Regulatory B cells improve ventricular remodeling after myocardial infarction by modulating monocyte migration
- PMID: 34302556
- PMCID: PMC8310480
- DOI: 10.1007/s00395-021-00886-4
Regulatory B cells improve ventricular remodeling after myocardial infarction by modulating monocyte migration
Abstract
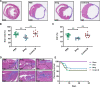
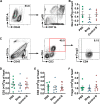
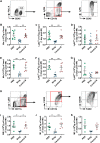
Overactivated inflammatory responses contribute to adverse ventricular remodeling after myocardial infarction (MI). Regulatory B cells (Bregs) are a newly discovered subset of B cells with immunomodulatory roles in many immune and inflammation-related diseases. Our study aims to determine whether the expansion of Bregs exerts a beneficial effect on ventricular remodeling and explore the mechanisms involved. Here, we showed that adoptive transfer of Bregs ameliorated ventricular remodeling in a murine MI model, as demonstrated by improved cardiac function, decreased scar size and attenuated interstitial fibrosis without changing the survival rate. Reduced Ly6Chi monocyte infiltration was found in the hearts of the Breg-transferred mice, while the infiltration of Ly6Clo monocytes was not affected. In addition, the replenishment of Bregs had no effect on the myocardial accumulation of T cells or neutrophils. Mechanistically, Bregs reduced the expression of C-C motif chemokine receptor 2 (CCR2) in monocytes, which inhibited proinflammatory monocyte recruitment to the heart from the peripheral blood and mobilization from the bone marrow. Breg-mediated protection against MI was abrogated by treatment with an interleukin 10 (IL-10) antibody. Finally, IL-10 neutralization reversed the effect of Bregs on monocyte migration and CCR2 expression. The present study suggests a therapeutic value of Bregs in limiting ventricular remodeling after MI through decreasing CCR2-mediated monocyte recruitment and mobilization.
Keywords: Interleukin 10; Monocytes; Myocardial infarction; Regulatory B cells; Ventricular remodeling.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures








Similar articles
-
Immune cells drive new immunomodulatory therapies for myocardial infarction: From basic to clinical translation.Front Immunol. 2023 Jan 24;14:1097295. doi: 10.3389/fimmu.2023.1097295. eCollection 2023. Front Immunol. 2023. PMID: 36761726 Free PMC article. Review.
-
Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice.Basic Res Cardiol. 2017 Jul;112(4):39. doi: 10.1007/s00395-017-0629-y. Epub 2017 May 22. Basic Res Cardiol. 2017. PMID: 28534119 Free PMC article.
-
Monocytes prime autoreactive T cells after myocardial infarction.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H116-H123. doi: 10.1152/ajpheart.00595.2019. Epub 2019 Dec 6. Am J Physiol Heart Circ Physiol. 2020. PMID: 31809213 Free PMC article.
-
Interleukin-37 and Dendritic Cells Treated With Interleukin-37 Plus Troponin I Ameliorate Cardiac Remodeling After Myocardial Infarction.J Am Heart Assoc. 2016 Dec 5;5(12):e004406. doi: 10.1161/JAHA.116.004406. J Am Heart Assoc. 2016. PMID: 27919929 Free PMC article.
-
The Role of Regulatory T Cells in Heart Repair After Myocardial Infarction.J Cardiovasc Transl Res. 2023 Jun;16(3):590-597. doi: 10.1007/s12265-022-10290-5. Epub 2023 Jun 22. J Cardiovasc Transl Res. 2023. PMID: 37347425 Review.
Cited by
-
Harnessing the Plasma Proteome to Mirror Current and Predict Future Cardiac Remodeling After Myocardial Infarction.J Cardiovasc Transl Res. 2023 Feb;16(1):3-16. doi: 10.1007/s12265-022-10326-w. Epub 2022 Oct 5. J Cardiovasc Transl Res. 2023. PMID: 36197585 Free PMC article.
-
Immunometabolism in heart failure.Nat Rev Cardiol. 2025 Jun 22. doi: 10.1038/s41569-025-01165-8. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40544171 Review.
-
Immune cells drive new immunomodulatory therapies for myocardial infarction: From basic to clinical translation.Front Immunol. 2023 Jan 24;14:1097295. doi: 10.3389/fimmu.2023.1097295. eCollection 2023. Front Immunol. 2023. PMID: 36761726 Free PMC article. Review.
-
Murine neonatal cardiac B cells promote cardiomyocyte proliferation and heart regeneration.NPJ Regen Med. 2023 Feb 11;8(1):7. doi: 10.1038/s41536-023-00282-7. NPJ Regen Med. 2023. PMID: 36774363 Free PMC article.
-
Ferroptosis in cardiovascular diseases: role and mechanism.Cell Biosci. 2023 Dec 15;13(1):226. doi: 10.1186/s13578-023-01169-2. Cell Biosci. 2023. PMID: 38102663 Free PMC article. Review.
References
-
- Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

