The Periaqueductal Gray and Its Extended Participation in Drug Addiction Phenomena
- PMID: 34302618
- PMCID: PMC8490541
- DOI: 10.1007/s12264-021-00756-y
The Periaqueductal Gray and Its Extended Participation in Drug Addiction Phenomena
Abstract
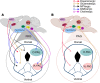
The periaqueductal gray (PAG) is a complex mesencephalic structure involved in the integration and execution of active and passive self-protective behaviors against imminent threats, such as immobility or flight from a predator. PAG activity is also associated with the integration of responses against physical discomfort (e.g., anxiety, fear, pain, and disgust) which occurs prior an imminent attack, but also during withdrawal from drugs such as morphine and cocaine. The PAG sends and receives projections to and from other well-documented nuclei linked to the phenomenon of drug addiction including: (i) the ventral tegmental area; (ii) extended amygdala; (iii) medial prefrontal cortex; (iv) pontine nucleus; (v) bed nucleus of the stria terminalis; and (vi) hypothalamus. Preclinical models have suggested that the PAG contributes to the modulation of anxiety, fear, and nociception (all of which may produce physical discomfort) linked with chronic exposure to drugs of abuse. Withdrawal produced by the major pharmacological classes of drugs of abuse is mediated through actions that include participation of the PAG. In support of this, there is evidence of functional, pharmacological, molecular. And/or genetic alterations in the PAG during the impulsive/compulsive intake or withdrawal from a drug. Due to its small size, it is difficult to assess the anatomical participation of the PAG when using classical neuroimaging techniques, so its physiopathology in drug addiction has been underestimated and poorly documented. In this theoretical review, we discuss the involvement of the PAG in drug addiction mainly via its role as an integrator of responses to the physical discomfort associated with drug withdrawal.
Keywords: Alcohol; Anti-reward circuit; Caffeine; Cannabis; Opioids; Periaqueductal gray; Reward circuit; Stimulants.
© 2021. Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Association AAP. Diagnostic and statistical manual of mental disorders: DSM-V. 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

