Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial
- PMID: 34302745
- PMCID: PMC8294807
- DOI: 10.1016/S2213-8587(21)00180-7
Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial
Abstract
Background: COVID-19 can lead to multiorgan failure. Dapagliflozin, a SGLT2 inhibitor, has significant protective benefits for the heart and kidney. We aimed to see whether this agent might provide organ protection in patients with COVID-19 by affecting processes dysregulated during acute illness.
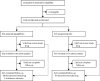
Methods: DARE-19 was a randomised, double-blind, placebo-controlled trial of patients hospitalised with COVID-19 and with at least one cardiometabolic risk factor (ie, hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and chronic kidney disease). Patients critically ill at screening were excluded. Patients were randomly assigned 1:1 to dapagliflozin (10 mg daily orally) or matched placebo for 30 days. Dual primary outcomes were assessed in the intention-to-treat population: the outcome of prevention (time to new or worsened organ dysfunction or death), and the hierarchial composite outcome of recovery (change in clinical status by day 30). Safety outcomes, in patients who received at least one study medication dose, included serious adverse events, adverse events leading to discontinuation, and adverse events of interest. This study is registered with ClinicalTrials.gov, NCT04350593.
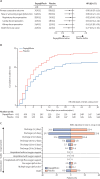
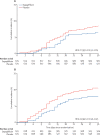
Findings: Between April 22, 2020 and Jan 1, 2021, 1250 patients were randomly assigned with 625 in each group. The primary composite outcome of prevention showed organ dysfunction or death occurred in 70 patients (11·2%) in the dapagliflozin group, and 86 (13·8%) in the placebo group (hazard ratio [HR] 0·80, 95% CI 0·58-1·10; p=0·17). For the primary outcome of recovery, 547 patients (87·5%) in the dapagliflozin group and 532 (85·1%) in the placebo group showed clinical status improvement, although this was not statistically significant (win ratio 1·09, 95% CI 0·97-1·22; p=0·14). There were 41 deaths (6·6%) in the dapagliflozin group, and 54 (8·6%) in the placebo group (HR 0·77, 95% CI 0·52-1·16). Serious adverse events were reported in 65 (10·6%) of 613 patients treated with dapagliflozin and in 82 (13·3%) of 616 patients given the placebo.
Interpretation: In patients with cardiometabolic risk factors who were hospitalised with COVID-19, treatment with dapagliflozin did not result in a statistically significant risk reduction in organ dysfunction or death, or improvement in clinical recovery, but was well tolerated.
Funding: AstraZeneca.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MA, EA, WKSB, ADMF, CRHF, AF, KG, RAG, CPJ, LNM, DDFM, JRLS, FT, SLW, OM, VC, RVPS, VG, PEL, FSS, and MP declare no competing interests. MNK has received a research grant for the conduct of this study from AstraZeneca. He has also received grant and research support from AstraZeneca. He has received a grant and honoraria from Boehringer-Ingelheim, and honoraria from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes), Janssen, Bayer, Novartis, Eli Lilly, and Vifor Pharma. OB reports grants from AstraZeneca, Novartis, Bayer, Amgen, Boehringer-Ingelheim, and Pfizer. GGK is the Principal Investigator of a biostatistics grant from AstraZeneca. He is also the Principal Investigator for biostatistics grants from other biopharmaceutical sponsors that have no relationship to the submitted work. SV reports receiving grants, speaker honoraria and consulting fees from Boehringer-Ingelheim, AstraZeneca, and Janssen. He has received speaker honoraria and consulting fees from Eli Lilly, and speaker honoraria from EOCI Pharmacomm Ltd, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group. He has also received grants and consulting fees from Amgen; grants, speaker honoraria and consulting fees from Bayer, and from Merck; grants from Bristol-Myers Squibb; speaker honoraria and consulting fees from HLS Therapeutics, Novo Nordisk, and Sanofi; and speaker honoraria from Novartis. AJ received research support for this study from AstraZeneca. He has stock options in DexCom, and has a pending patent for fusion protein nanodiscs for the treatment of heart failure. RF reports research grants and personal fees from AstraZeneca, Bayer and Servier; and research grants from Pfizer, EMS, Aché, Brazilian Ministry of Health, University Health Network, and Lemann Foundation Reseach Fellowship. MN is a consultant for Roche, Vifor, and Amgen, and has received speaking honoraria from Abbott. RE, JO, SBG, JB, AML, and PA are employees and stockholders of AstraZeneca.
Figures
Comment in
-
Dapagliflozin in patients with COVID-19: truth or dare.Lancet Diabetes Endocrinol. 2021 Sep;9(9):550-551. doi: 10.1016/S2213-8587(21)00206-0. Epub 2021 Jul 21. Lancet Diabetes Endocrinol. 2021. PMID: 34302746 Free PMC article. No abstract available.
-
Dapagliflozin in patients with COVID-19: mind the kidneys.Lancet Diabetes Endocrinol. 2022 Feb;10(2):97-98. doi: 10.1016/S2213-8587(21)00329-6. Epub 2021 Dec 15. Lancet Diabetes Endocrinol. 2022. PMID: 34921752 Free PMC article. No abstract available.
-
Dapagliflozin in patients with COVID-19: mind the kidneys - Authors' reply.Lancet Diabetes Endocrinol. 2022 Feb;10(2):98-99. doi: 10.1016/S2213-8587(21)00326-0. Epub 2021 Dec 15. Lancet Diabetes Endocrinol. 2022. PMID: 34921753 Free PMC article. No abstract available.
References
-
- Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2021;19:345–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

