BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study
- PMID: 34302788
- PMCID: PMC8395368
- DOI: 10.1016/S1474-4422(21)00174-5
BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study
Abstract
Background: Progressive multifocal leukoencephalopathy, a rare disease of the CNS caused by JC virus and occurring in immunosuppressed people, is typically fatal unless adaptive immunity is restored. JC virus is a member of the human polyomavirus family and is closely related to the BK virus. We hypothesised that use of partly HLA-matched donor-derived BK virus-specific T cells for immunotherapy in progressive multifocal leukoencephalopathy would be feasible and safe.
Methods: We did an open-label, single-cohort pilot study in patients (aged 18 years or older) with clinically definite progressive multifocal leukoencephalopathy and disease progression in the previous month at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD, USA). Overlapping peptide libraries derived from large T antigen and major capsid protein VP1 of BK virus with high sequence homology to JC virus counterparts were used to generate polyomavirus-specific T cells cross-recognising JC virus antigens. Polyomavirus-specific T cells were manufactured from peripheral blood mononuclear cells of first-degree relative donors aged 18 years or older. These cells were administered to patients by intravenous infusion at 1 × 106 polyomavirus-specific T cells per kg, followed by up to two additional infusions at 2 × 106 polyomavirus-specific T cells per kg. The primary endpoints were feasibility (no manufacturing failure based on meeting release criteria, achieving adequate numbers of cell product for clinical use, and showing measurable antiviral activity) and safety in all patients. The safety monitoring period was 28 days after each infusion. Patients were followed up with serial MRI for up to 12 months after the final infusion. This trial is registered at ClinicalTrials.gov, NCT02694783.
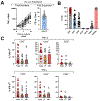
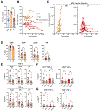
Findings: Between April 7, 2016, and Oct 19, 2018, 26 patients were screened, of whom 12 were confirmed eligible and received treatment derived from 14 matched donors. All administered polyomavirus-specific T cells met the release criteria and recognised cognate antigens in vitro. 12 patients received at least one infusion, ten received at least two, and seven received a total of three infusions. The median on-study follow-up was 109·5 days (range 23-699). All infusions were tolerated well, and no serious treatment-related adverse events were observed. Seven patients survived progressive multifocal leukoencephalopathy for longer than 1 year after the first infusion, whereas five died of progressive multifocal leukoencephalopathy within 3 months.
Interpretation: We showed that generation of polyomavirus-specific T cells from healthy related donors is feasible, and these cells can be safely used as an infusion for adoptive immunotherapy of progressive multifocal leukoencephalopathy. Although not powered to assess efficacy, our data provide additional support for this strategy as a potential life-saving therapy for some patients.
Funding: Intramural Research Program of the National Institute of Neurological Disorders and Stroke of the NIH.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests IC reports providing free consultative advice to Cellevolve and is a shareholder in Nouscom AG and Keires AG, outside the submitted work. DSR reports non-financial support from Biogen, outside the submitted work. In addition, DSR has two patents issued (System and Method of Automatically Detecting Tissue Abnormalities [US patent 9607392]; and Method of Analyzing Multi-Sequence MRI Data for Analyzing Brain Abnormalities in a Subject [US patent 9888876]). PM reports having received consultation fees from ATARA Biological and AstraZeneca, outside the submitted work. JB is a member of the data safety monitoring board for the AMADEUS trial (azacitidine after a stem cell transplant). All other authors declare no competing interests.
Figures


Comment in
-
Hope for progressive multifocal leukoencephalopathy.Lancet Neurol. 2021 Aug;20(8):589-591. doi: 10.1016/S1474-4422(21)00206-4. Lancet Neurol. 2021. PMID: 34302776 No abstract available.
References
-
- Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DWG, et al.Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003September;71(1):115–23. - PubMed
-
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al.Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009March15;199(6):837–46. - PubMed
-
- Johnson EM, Wortman MJ, Dagdanova AV, Lundberg PS, Daniel DC. Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy. Clin Dev Immunol. 2013;2013(4):197807–10. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

