Diagnostic accuracy of fresh drooled saliva for SARS-CoV-2 in travelers
- PMID: 34302954
- PMCID: PMC8294709
- DOI: 10.1016/j.tmaid.2021.102144
Diagnostic accuracy of fresh drooled saliva for SARS-CoV-2 in travelers
Abstract
Background: The standard for SARS-CoV-2 diagnosis is RT-PCR from nasopharyngeal or oropharyngeal swabs. Major airports require COVID-19 screening, and saliva has the potential as a substitute specimen for SARS-CoV-2 diagnosis. We investigated the utility of fresh drooled saliva against NPS for COVID-19 screening of travelers.
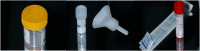
Methods: We recruited 81 travelers and 15 non-travelers (including ten controls) prospectively within a mean of 3·22 days of RT-PCR confirmed COVID-19. Each study participant provided 2 mls of early morning fresh drooled whole saliva separately into a sterile plastic container and GeneFiX™ saliva collection kit. The saliva specimens were processed within 4 h and tested for SARS-CoV-2 genes (E, RdRP, and N2) and the results compared to paired NPS RT-PCR for diagnostic accuracy.
Results: Majority of travellers were asymptomatic (75·0%) with a mean age of 34·26 years. 77 travelers were RT-PCR positive at the time of hospitalization whilst three travelers had positive contacts. In this group, the detection rate for SARS-CoV-2 with NPS, whole saliva, and GeneFiX™ were comparable (89·3%, 50/56; 87·8%, 43/49; 89·6%, 43/48). Both saliva collection methods were in good agreement (Kappa = 0·69). There was no statistical difference between the detection rates of saliva and NPS (p > 0·05). Detection was highest for the N2 gene whilst the E gene provided the highest viral load (mean = 27·96 to 30·10, SD = 3·14 to 3·85). Saliva specimens have high sensitivity (80·4%) and specificity (90·0%) with a high positive predictive value of 91·8% for SARS-CoV-2 diagnosis.
Conclusion: Saliva for SARS-CoV-2 screening is a simple accurate technique comparable with NPS RT-PCR.
Keywords: Aviation; COVID-19; Ct. value; RT-PCR; Travel medicine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures



References
-
- World Health Organization (WHO) Coronavirus [internet]. 2021. 2020. https://www.who.int/health-topics/coronavirus#tab=tab_1
-
- World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard [internet] https://covid19.who.int
-
- COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) [internet]https://www.covid19treatmentguidelines.nih.gov// - PubMed
-
- Relman R.D.A., Choffnes E.R., Mack A. Infectious disease movement in a borderless World. Infect Dis Mov a Borderless World. 2010
-
- Juniac A De. Annual review [internet] 2020. https://online.fliphtml5.com/uzvev/hfuf/?_cldee=MTQzNTg1NDk5NUBxcS5jb20%...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

