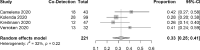Identification of bacterial co-detections in COVID-19 critically Ill patients by BioFire® FilmArray® pneumonia panel: a systematic review and meta-analysis
- PMID: 34303085
- PMCID: PMC8245667
- DOI: 10.1016/j.diagmicrobio.2021.115476
Identification of bacterial co-detections in COVID-19 critically Ill patients by BioFire® FilmArray® pneumonia panel: a systematic review and meta-analysis
Abstract
Among critically ill COVID-19 patients, bacterial coinfections may occur, and timely appropriate therapy may be limited with culture-based microbiology due to turnaround time and diagnostic yield challenges (e.g. antibiotic pre-exposure). We performed a systematic review and meta-analysis of the impact of BioFire® FilmArray® Pneumonia Panel in detecting bacteria and clinical management among critically ill COVID-19 patients admitted to the ICU. Seven studies with 558 patients were included. Antibiotic use before respiratory sampling occurred in 28-79% of cases. The panel incidence of detections was 33% (95% CI 0.25 to 0.41, I2=32%) while culture yielded 18% (95% CI 0.02 to 0.45; I2=93%). The panel was associated with approximately a 1 and 2 day decrease in turnaround for identification and common resistance targets, respectively. The panel may be an important tool for clinicians to improve antimicrobial use in critically ill COVID-19 patients.
Keywords: Bacterial; BioFire Pneumonia Panel; COVID-19; Pneumonia; SARS-CoV-2.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest T.T. Timbrook and K.D. Hueth are employees of BioFire Diagnostics; C.C. Ginocchio is an employee of bioMérieux and BioFire Diagnostics. Authors report no other conflicts of interest.
Figures
References
-
- Buchan BW, Windham S, Balada-Llasat JM, Leber A, Harrington A, Relich R, et al. Practical comparison of the BioFire FilmArray pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol. 2020;58(7) - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous