SARS-CoV-2 attenuates corticosteroid sensitivity by suppressing DUSP1 expression and activating p38 MAPK pathway
- PMID: 34303662
- PMCID: PMC8295491
- DOI: 10.1016/j.ejphar.2021.174374
SARS-CoV-2 attenuates corticosteroid sensitivity by suppressing DUSP1 expression and activating p38 MAPK pathway
Abstract
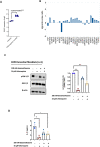
The efficacy of corticosteroids and its use for the treatment of SARS-CoV-2 infections is controversial. In this study, using data sets of SARS-CoV-2 infected lung tissues and nasopharyngeal swabs, as well as in vitro experiments, we show that SARS-CoV-2 infection significantly downregulates DUSP1 expression. This downregulation of DUSP1 could be the mechanism regulating the enhanced activation of MAPK pathway as well as the reported steroid resistance in SARS-CoV-2 infection. Moreover, chloroquine, an off labeled COVID-19 drug is able to induce DUSP1 and attenuate MAPK pathway; and is expected to improve sensitivity to steroid treatment. However, further mechanistic studies are required to confirm this effect.
Keywords: COVID-19; Chloroquine; Corticosteroid; DUSP1; SARS-CoV-2; p38 MAPK pathway.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. - PubMed
-
- Awasthi S., Wagner T., Venkatakrishnan A.J., Puranik A., Hurchik M., Agarwal V., Conrad I., Kirkup C., Arunachalam R., O'Horo J., Kremers W., Kashyap R., Morice W., Halamka J., Williams A.W., Faubion W.A., Badley A.D., Gores G.J., Soundararajan V. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discovery. 2021;7:55. - PMC - PubMed
-
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. - PubMed
-
- Barnes P.J. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006;27:413–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

