Evaluation of a multiplexed coronavirus antigen array for detection of SARS-CoV-2 specific IgG in COVID-19 convalescent plasma
- PMID: 34303688
- PMCID: PMC8297968
- DOI: 10.1016/j.jim.2021.113104
Evaluation of a multiplexed coronavirus antigen array for detection of SARS-CoV-2 specific IgG in COVID-19 convalescent plasma
Abstract
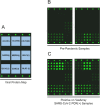
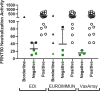
Mitigation of the COVID-19 pandemic requires an understanding of the antibody response to SARS-CoV-2. However, throughout the development of SARS-CoV-2 IgG antibody assays during the past year, cross-reactivity to other coronaviruses remained a question. To address these issues, we evaluated IgG in COVID-19 convalescent plasma samples for reactivity against three SARS-CoV-2 antigens including full-length spike, receptor binding domain, and the proximal extracellular fusion domain, and spike antigens from other coronaviruses (SARS-CoV, MERS-CoV, hCoV-HKU1, hCoV-OC43, hCoV-229E and hCoV-NL63) using the VaxArray Coronavirus SeroAssay which is a multiplexed antigen assay developed by InDevR Inc. These results were compared to two commercial SARS-CoV-2 IgG ELISAs targeting either the SARS-CoV-2 nucleocapsid or spike antigens and a live virus focus reduction neutralizing antibody test (FRNT). The VaxArray platform showed high specificity for detection of SARS-CoV-2 IgG, evident from lack of reactivity to SARS-CoV-2 antigens despite significant reactivity to endemic coronavirus antigens in pre-pandemic samples. SARS-CoV-2 IgG positive samples reacted weakly to SARS-CoV spike but not to MERS-CoV. While the VaxArray platform had overall comparable results to the spike and nucleocapsid IgG ELISAs, results were more similar to the spike antigen ELISA and the platform displayed a higher sensitivity and specificity than both ELISAs. Samples with FRNT titers below 1/23 reported negative on VaxArray, while positive samples on VaxArray had significantly higher neutralizing antibody titers. These results suggest that the VaxArray Coronavirus SeroAssay performs with high sensitivity and specificity for the detection of SARS-CoV-2 IgG, and positive results on the platform indicate SARS-CoV-2 neutralizing activity.
Keywords: Antigen array; COVID-19; SARS-CoV-2; SARS-CoV-2 IgG; Spike antigen.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Kathy L. Rowlen and Erica D. Dawson are stockholders of InDevR, Inc. All other authors declare that they have no conflicts of interest.
Figures

References
-
- Abbasi J. COVID-19 antibody tests perform well in head-to-head comparison. JAMA. 2020;324(18):1818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

