Micronutrients for Attention-Deficit/Hyperactivity Disorder in Youths: A Placebo-Controlled Randomized Clinical Trial
- PMID: 34303786
- PMCID: PMC8782920
- DOI: 10.1016/j.jaac.2021.07.005
Micronutrients for Attention-Deficit/Hyperactivity Disorder in Youths: A Placebo-Controlled Randomized Clinical Trial
Erratum in
-
Correction.J Am Acad Child Adolesc Psychiatry. 2022 Aug;61(8):1066. doi: 10.1016/j.jaac.2022.04.021. Epub 2022 May 6. J Am Acad Child Adolesc Psychiatry. 2022. PMID: 35533797 No abstract available.
-
Correction.J Am Acad Child Adolesc Psychiatry. 2023 May;62(5):607. doi: 10.1016/j.jaac.2022.12.009. Epub 2022 Dec 28. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 36586665 No abstract available.
-
Correction.J Am Acad Child Adolesc Psychiatry. 2023 Nov;62(11):1276. doi: 10.1016/j.jaac.2023.07.995. Epub 2023 Aug 3. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 37543079 No abstract available.
Abstract
Objective: To evaluate whether micronutrients (vitamins/minerals) benefit attention-deficit/hyperactivity disorder (ADHD) and irritability in a North American pediatric sample.
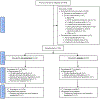
Method: A 3-site, 8-week, placebo-controlled, randomized clinical trial of micronutrients was conducted in nonmedicated children aged 6 to 12 years with ADHD and at least 1 impairing irritability symptom by parent report on the Child and Adolescent Symptom Inventory-5 (CASI-5). A priori-defined primary outcomes were Clinical Global Impression-Improvement (CGI-I) (CGI-I of 1 or 2 = treatment responder) and parent-rated CASI-5 composite score of ADHD, oppositional defiant, disruptive mood dysregulation, and peer conflict symptoms, including impairment scores.
Results: Of 135 randomized (mean age 9.8 years), 126 youths (93%) comprised the modified intention-to-treat population. Blinding was maintained. For the CGI-I, 54% of the micronutrient and 18% of the placebo group were responders (risk ratio = 2.97, 97.5% CI = 1.50, 5.90, p < .001). CASI-5 composite scores improved significantly for both groups (p < .01), with a mean change of -0.31 (95% CI = -0.39, -0.23) in the micronutrient group and a mean change of -0.28 (95% CI = -0.38, -0.19) in the placebo group. However, the between-group difference was not significant (mean change = -0.02; 97.5% CI = -0.16, 0.12, effect size = 0.07, p = .70). The micronutrient group grew 6 mm more than the placebo group (p = .002). No serious adverse events or clinically significant changes from baseline in blood and urine tests occurred.
Conclusion: Micronutrients showed global benefit over placebo by blinded clinician rating, but not by parent-report CASI-5 composite rating in a population with ADHD and irritability. Micronutrients showed greater height growth. Micronutrients were well tolerated, and the majority of participants adhered to the number of capsules prescribed. This randomized controlled trial replicates safety and efficacy reported for ADHD in 2 smaller trials of a similar formula containing all vitamins and known essential minerals in amounts between the Recommended Dietary Allowance and Upper Tolerable Intake Level.
Clinical trial registration information: Micronutrients for ADHD in Youth (MADDY) Study; https://clinicaltrials.gov; NCT03252522.
Keywords: attention-deficit/hyperactivity disorder; dysregulated mood; irritability; micronutrients.
Copyright © 2021 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure: Dr. Gracious has been or is a consultant to AstraZeneca, Otsuka, and Novo Nordisk. Dr. Arnold has received research funding from Forest, Eli Lilly and Co., Noven, Shire (a Takeda company), Supernus, Roche, YoungLiving, NIH, and Autism Speaks; has consulted with Pfizer, Tris Pharma, and Waypoint; and has been on advisory boards for Arbor, Ironshore, Otsuka, Pfizer, Roche, Seaside Therapeutics, and Shire. Drs. Johnstone, Hatsu, Eiterman, Bruton, Ast, Hughes, Leung and Mss. Tost, Srikanth, Robinette, Stern, Millington have reported no biomedical financial interests or potential conflicts of interest.
Figures
Comment in
-
Editorial: Accumulating Evidence for the Benefit of Micronutrients for Children With Attention-Deficit/Hyperactivity Disorder.J Am Acad Child Adolesc Psychiatry. 2022 May;61(5):599-600. doi: 10.1016/j.jaac.2021.08.008. Epub 2021 Aug 17. J Am Acad Child Adolesc Psychiatry. 2022. PMID: 34416292
-
Dr. Johnstone et al. Reply to Dr. Elmrayed.J Am Acad Child Adolesc Psychiatry. 2023 Nov;62(11):1171-1175. doi: 10.1016/j.jaac.2023.07.994. Epub 2023 Aug 3. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 37543077 Free PMC article.
-
Blinding of Participants to Treatment and Implications for Assessments of Efficacy in Attention-Deficit/Hyperactivity Disorder Micronutrient Study.J Am Acad Child Adolesc Psychiatry. 2023 Nov;62(11):1167-1168. doi: 10.1016/j.jaac.2022.12.029. Epub 2023 Aug 3. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 37543078 No abstract available.
-
Dr. Johnstone et al. Reply to Dr. Hamilton.J Am Acad Child Adolesc Psychiatry. 2023 Nov;62(11):1168-1170. doi: 10.1016/j.jaac.2023.07.992. Epub 2023 Aug 3. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 37543080 Free PMC article.
-
Dosages of Nutrient Supplements and Potential Long-Term Toxicity in Attention-Deficit/Hyperactivity Disorder Micronutrient Study.J Am Acad Child Adolesc Psychiatry. 2023 Nov;62(11):1170-1171. doi: 10.1016/j.jaac.2023.01.027. Epub 2023 Aug 3. J Am Acad Child Adolesc Psychiatry. 2023. PMID: 37543081
References
-
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-5. American Psychiatric Association. 2013; Arlington, VA: American Psychiatric Publishing.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical