Metastatic inflammatory breast cancer: survival outcomes and prognostic factors in the national, multicentric, and real-life French cohort (ESME)
- PMID: 34303929
- PMCID: PMC8327489
- DOI: 10.1016/j.esmoop.2021.100220
Metastatic inflammatory breast cancer: survival outcomes and prognostic factors in the national, multicentric, and real-life French cohort (ESME)
Abstract
Background: Primary inflammatory breast cancer (IBC) is a rare and aggressive entity whose prognosis has been improved by multimodal therapy. However, 5-year overall survival (OS) remains poor. Given its low incidence, the prognosis of IBC at metastatic stage is poorly described.
Materials and methods: This study aimed to compare OS calculated from the diagnosis of metastatic disease between IBC patients and non-IBC patients in the Epidemiological Strategy and Medical Economics database (N = 16 702 patients). Secondary objectives included progression-free survival (PFS) after first-line metastatic treatment, identification of prognostic factors for OS and PFS, and evolution of survival during the study period.
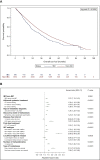
Results: From 2008 to 2014, 7465 patients with metastatic breast cancer and known clinical status of their primary tumor (T) were identified (582 IBC and 6883 non-IBC). Compared with metastatic non-IBC, metastatic IBC was associated with less hormone receptor-positive (44% versus 65.6%), more human epidermal growth factor receptor 2-positive (30% versus 18.6%), and more triple-negative (25.9% versus 15.8%) cases, more frequent de novo M1 stage (53.3% versus 27.7%; P < 0.001), and shorter median disease-free interval (2.02 years versus 4.9 years; P < 0.001). With a median follow-up of 50.2 months, median OS was 28.4 months [95% confidence interval (CI) 24.1-33.8 months] versus 37.2 months (95% CI 36.1-38.5 months) in metastatic IBC and non-IBC cases, respectively (P < 0.0001, log-rank test). By multivariate analysis, OS was significantly shorter in the metastatic IBC group compared with the metastatic non-IBC group [hazard ratio = 1.27 (95% CI 1.1-1.4); P = 0.0001]. Survival of metastatic IBC patients improved over the study period: median OS was 24 months (95% CI 20-31.9 months), 29 months (95% CI 21.7-39.9 months), and 36 months (95% CI 27.9-not estimable months) if diagnosis of metastatic disease was carried out until 2010, between 2011 and 2012, and from 2013, respectively (P = 0.003).
Conclusion: IBC is independently associated with adverse outcome when compared with non-IBC in the metastatic setting.
Keywords: inflammatory breast cancer; metastatic breast cancer; multimodal therapy; prognostic factors; real-life study.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure MC declares the following: Advisory board: AstraZeneca, Novartis, Abbvie, Sanofi, Pfizer, Sandoz, ACCORD, and Lilly GT1 group; consultant: Pierre Fabre Oncology, Sanofi, Novartis, and Servier; speaker bureau: Novartis; travel: Pfizer, Novartis, Roche, and AstraZeneca. BP declares the following: Consulting/advisor: Puma Biotechnology, Novartis, Myriad Genetics, and Pierre Fabre; personal fees: Novartis, AstraZeneca, MSD Oncology, and Pfizer; research funding: Daiichi, Puma Biotechnology, Novartis, Merus, Pfizer, and AstraZeneca. AG reports non-financial support from AstraZeneca, Roche, Pfizer, and Novartis. TdLMR reports grants, personal fees, and non-financial support from Pfizer; grants and non-financial support from Novartis and MSD; personal fees and non-financial support from AstraZeneca, Roche Genentech, and TESARO-GSK; and personal fees from CLOVIS ONCOLOGY and MYLAN, outside the submitted work. The remaining authors have declared no conflicts of interest.
Figures
References
-
- Giuliano A.E., Connolly J.L., Edge S.B. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. - PubMed
-
- Robertson F.M., Bondy M., Yang W. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–375. - PubMed
-
- Wingo P.A., Jamison P.M., Young J.L., Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15:321–328. - PubMed
-
- Curcio L.D., Rupp E., Williams W.L. Beyond palliative mastectomy in inflammatory breast cancer–a reassessment of margin status. Ann Surg Oncol. 1999;6:249–254. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

