Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France
- PMID: 34304047
- PMCID: PMC8299153
- DOI: 10.1016/j.ebiom.2021.103495
Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France
Abstract
Background: Children are underrepresented in the COVID-19 pandemic and often experience milder disease than adolescents and adults. Reduced severity is possibly due to recent and more frequent seasonal human coronaviruses (HCoV) infections. We assessed the seroprevalence of SARS-CoV-2 and seasonal HCoV specific antibodies in a large cohort in north-eastern France.
Methods: In this cross-sectional seroprevalence study, serum samples were collected from children and adults requiring hospital admission for non-COVID-19 between February and August 2020. Antibody responses to SARS-CoV-2 and seasonal HCoV (229E, HKU1, NL63, OC43) were assessed using a bead-based multiplex assay, Luciferase-Linked ImmunoSorbent Assay, and a pseudotype neutralisation assay.
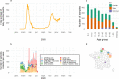
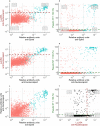
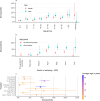
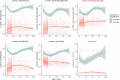
Findings: In 2,408 individuals, seroprevalence of SARS-CoV-2-specific antibodies was 7-8% with three different immunoassays. Antibody levels to seasonal HCoV increased substantially up to the age of 10. Antibody responses in SARS-CoV-2 seropositive individuals were lowest in adults 18-30 years. In SARS-CoV-2 seronegative individuals, we observed cross-reactivity between antibodies to the four HCoV and SARS-CoV-2 Spike. In contrast to other antibodies to SARS-CoV-2, specific antibodies to sub-unit 2 of Spike (S2) in seronegative samples were highest in children. Upon infection with SARS-CoV-2, antibody levels to Spike of betacoronavirus OC43 increased across the whole age spectrum. No SARS-CoV-2 seropositive individuals with low levels of antibodies to seasonal HCoV were observed.
Interpretation: Our findings underline significant cross-reactivity between antibodies to SARS-CoV-2 and seasonal HCoV, but provide no significant evidence for cross-protective immunity to SARS-CoV-2 infection due to a recent seasonal HCoV infection. In particular, across all age groups we did not observe SARS-CoV-2 infected individuals with low levels of antibodies to seasonal HCoV.
Funding: This work was supported by the « URGENCE COVID-19 » fundraising campaign of Institut Pasteur, by the French Government's Investissement d'Avenir program, Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases (Grant No. ANR-10-LABX-62-IBEID), and by the REACTing (Research & Action Emerging Infectious Diseases), and by the RECOVER project funded by the European Union's Horizon 2020 research and innovation programme under grant agreement No. 101003589, and by a grant from LabEx IBEID (ANR-10-LABX-62-IBEID).
Keywords: COVID-19; SARS-CoV-2; antibody response; seasonal coronaviruses; sero-epidemiology; seroprevalence.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest MTW and SPel are inventors on provisional patent PCT/US 63/057.471 on a serological antibody-based diagnostics of SARS-CoV-2 infection. Dr. Dubos reports grants from Universite de Lille, during the conduct of the study. Dr. van der WERF reports grants from Agence Nationale de la Recherche, grants from European Union's Horizon 2020 research and innovation programme, during the conduct of the study; In addition, Dr. van der WERF has a patent USE OF PROTEINS AND PEPTIDES CODED BY THE GENOME OF A NOVEL STRAIN OF SARS ASSOCIATED CORONAVIRUS issued, and a patent SEVERE ACUTE RESPIRATORY SYNDROME (SARS) - ASSOCIATED CORONAVIRUS DIAGNOSTICS pending.
Figures

Similar articles
-
Prior infection by seasonal coronaviruses, as assessed by serology, does not prevent SARS-CoV-2 infection and disease in children, France, April to June 2020.Euro Surveill. 2021 Apr;26(13):2001782. doi: 10.2807/1560-7917.ES.2021.26.13.2001782. Euro Surveill. 2021. PMID: 33797390 Free PMC article.
-
Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study.Emerg Microbes Infect. 2021 Dec;10(1):664-676. doi: 10.1080/22221751.2021.1905488. Emerg Microbes Infect. 2021. PMID: 33734013 Free PMC article.
-
Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post-COVID-19 Convalescent Samples.J Infect Dis. 2021 Oct 28;224(8):1305-1315. doi: 10.1093/infdis/jiab333. J Infect Dis. 2021. PMID: 34161567 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
Heterologous humoral immunity to human and zoonotic coronaviruses: Aiming for the achilles heel.Semin Immunol. 2021 Jun;55:101507. doi: 10.1016/j.smim.2021.101507. Epub 2021 Oct 25. Semin Immunol. 2021. PMID: 34716096 Free PMC article. Review.
Cited by
-
No substantial preexisting B cell immunity against SARS-CoV-2 in healthy adults.iScience. 2022 Mar 18;25(3):103951. doi: 10.1016/j.isci.2022.103951. Epub 2022 Feb 19. iScience. 2022. PMID: 35224466 Free PMC article.
-
Microarray-Based Detection of Antibodies against SARS-CoV-2 Proteins, Common Respiratory Viruses and Type I Interferons.Viruses. 2021 Dec 20;13(12):2553. doi: 10.3390/v13122553. Viruses. 2021. PMID: 34960822 Free PMC article.
-
Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study.Int J Infect Dis. 2022 Mar;116:47-50. doi: 10.1016/j.ijid.2021.12.354. Epub 2021 Dec 26. Int J Infect Dis. 2022. PMID: 34965462 Free PMC article.
-
Seroprevalence of four endemic human coronaviruses and, reactivity and neutralization capability against SARS-CoV-2 among children in the Philippines.Sci Rep. 2023 Feb 9;13(1):2310. doi: 10.1038/s41598-023-29072-3. Sci Rep. 2023. PMID: 36759702 Free PMC article.
-
Multiplex Technologies in COVID-19 Research, Diagnostics, and Prognostics: Battling the Pandemic.Methods Mol Biol. 2022;2511:3-20. doi: 10.1007/978-1-0716-2395-4_1. Methods Mol Biol. 2022. PMID: 35838948 Review.
References
-
- Organization WH. Coronavirus disease 2019 (COVID-19): situation report. 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

