Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems
- PMID: 34304387
- PMCID: PMC8310561
- DOI: 10.1007/s13770-021-00373-w
Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems
Abstract
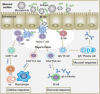
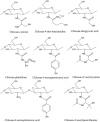
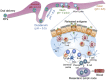
Vaccination has been recently attracted as one of the most successful medical treatments of the prevalence of many infectious diseases. Mucosal vaccination has been interested in many researchers because mucosal immune responses play part in the first line of defense against pathogens. However, mucosal vaccination should find out an efficient antigen delivery system because the antigen should be protected from degradation and clearance, it should be targeted to mucosal sites, and it should stimulate mucosal and systemic immunity. Accordingly, mucoadhesive polymeric particles among the polymeric particles have gained much attention because they can protect the antigen from degradation, prolong the residence time of the antigen at the target site, and control the release of the loaded vaccine, and results in induction of mucosal and systemic immune responses. In this review, we discuss advances in the development of several kinds of mucoadhesive polymeric particles for mucosal vaccine delivery.
Keywords: Antigen delivery; Mucoadhesive polymers; Mucosal vaccination; Polymeric particles.
© 2021. The Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
