Degradation of Premature-miR-181b by the Translin/Trax RNase Increases Vascular Smooth Muscle Cell Stiffness
- PMID: 34304585
- PMCID: PMC8363557
- DOI: 10.1161/HYPERTENSIONAHA.120.16690
Degradation of Premature-miR-181b by the Translin/Trax RNase Increases Vascular Smooth Muscle Cell Stiffness
Abstract
[Figure: see text].
Keywords: aorta; cardiovascular diseases; microRNA degradation; risk factors; vascular smooth muscle; vasopressin.
Figures


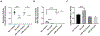
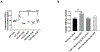
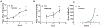
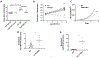
References
-
- O’Rourke M Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–347 - PubMed
-
- O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658 - PubMed
-
- Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in ckd and esrd. Kidney Int. 2012;82:388–400 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

