Expanding the classical paradigm: what we have learnt from vertebrates about sex chromosome evolution
- PMID: 34304593
- PMCID: PMC8310716
- DOI: 10.1098/rstb.2020.0097
Expanding the classical paradigm: what we have learnt from vertebrates about sex chromosome evolution
Abstract
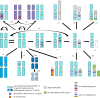
Until recently, the field of sex chromosome evolution has been dominated by the canonical unidirectional scenario, first developed by Muller in 1918. This model postulates that sex chromosomes emerge from autosomes by acquiring a sex-determining locus. Recombination reduction then expands outwards from this locus, to maintain its linkage with sexually antagonistic/advantageous alleles, resulting in Y or W degeneration and potentially culminating in their disappearance. Based mostly on empirical vertebrate research, we challenge and expand each conceptual step of this canonical model and present observations by numerous experts in two parts of a theme issue of Phil. Trans. R. Soc. B. We suggest that greater theoretical and empirical insights into the events at the origins of sex-determining genes (rewiring of the gonadal differentiation networks), and a better understanding of the evolutionary forces responsible for recombination suppression are required. Among others, crucial questions are: Why do sex chromosome differentiation rates and the evolution of gene dose regulatory mechanisms between male versus female heterogametic systems not follow earlier theory? Why do several lineages not have sex chromosomes? And: What are the consequences of the presence of (differentiated) sex chromosomes for individual fitness, evolvability, hybridization and diversification? We conclude that the classical scenario appears too reductionistic. Instead of being unidirectional, we show that sex chromosome evolution is more complex than previously anticipated and principally forms networks, interconnected to potentially endless outcomes with restarts, deletions and additions of new genomic material. This article is part of the theme issue 'Challenging the paradigm in sex chromosome evolution: empirical and theoretical insights with a focus on vertebrates (Part II)'.
Keywords: evolution; sex chromosomes; sex determination; vertebrates.
Figures
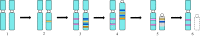
References
-
- Ohno S. 1967. Sex chromosomes and sex-linked genes. Berlin, Germany: Springer-Verlag.
-
- Fisher RA. 1931. The evolution of dominance. Biol. Rev. 6, 345-368.
