Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals
- PMID: 34304742
- PMCID: PMC8310732
- DOI: 10.1186/s12916-021-02055-9
Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals
Abstract
Background: The global pandemic of coronavirus disease 2019 (COVID-19) is caused by infection with the SARS-CoV-2 virus. Currently, there are three approved vaccines against SARS-CoV-2 in the USA, including two based on messenger RNA (mRNA) technology that has demonstrated high vaccine efficacy. We sought to characterize humoral immune responses, at high resolution, during immunization with the BNT162b2 (Pfizer-BioNTech) vaccine in individuals with or without prior history of natural SARS-CoV-2 infection.
Methods: We determined antibody responses after each dose of the BNT162b2 SARS-CoV-2 vaccine in individuals who had no prior history of SARS-CoV-2 infection (seronegative) and individuals that had previous viral infection 30-60 days prior to first vaccination (seropositive). To do this, we used both an antibody isotype-specific multiplexed bead-based binding assays targeting multiple SARS-CoV-2 viral protein antigens and an assay that identified potential SARS-CoV-2 neutralizing antibody levels. Moreover, we mapped antibody epitope specificity after immunization using SARS-CoV-2 spike protein peptide arrays.
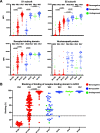
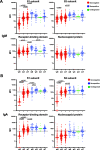
Results: Antibody levels were significantly higher after a single dose in seropositive individuals compared to seronegative individuals and were comparable to levels observed in seronegative individuals after two doses. While IgG was boosted by vaccination for both seronegative and seropositive individuals, only seronegative individuals had increased IgA or IgM antibody titers after primary immunization. We identified immunodominant peptides targeted on both SARS-CoV-2 spike S1 and S2 subunits after vaccination.
Conclusion: These findings demonstrated the antibody responses to SARS-CoV-2 immunization in seropositive and seronegative individuals and provide support for the concept of using prior infection history as a guide for the consideration of future vaccination regimens. Moreover, we identified key epitopes on the SARS-CoV-2 spike protein that are targeted by antibodies after vaccination that could guide future vaccine and immune correlate development.
Keywords: Antibody response; SARS-CoV-2; mRNA vaccine.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang XL, Tian WG, Hu L, Zhang XX, Xiang JL, du HX, Liu HW, Lang CH, Luo XH, Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ, Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC, Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. - DOI - PubMed
-
- Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG, Overcoming COVID-19 Investigators. CDC COVID-19 Response Team Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. - DOI - PMC - PubMed
-
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

