eIF2-dependent translation initiation: Memory consolidation and disruption in Alzheimer's disease
- PMID: 34304995
- PMCID: PMC8782933
- DOI: 10.1016/j.semcdb.2021.07.009
eIF2-dependent translation initiation: Memory consolidation and disruption in Alzheimer's disease
Abstract
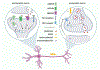
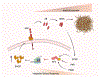
Memory storage is a conserved survivability feature, present in virtually any complex species. During the last few decades, much effort has been devoted to understanding how memories are formed and which molecular switches define whether a memory should be stored for a short or a long period of time. Among these, de novo protein synthesis is known to be required for the conversion of short- to long-term memory. There are a number translational control pathways involved in synaptic plasticity and memory consolidation, including the phosphorylation of the eukaryotic initiation factor 2 alpha (eIF2α), which has emerged as a critical molecular switch for long-term memory consolidation. In this review, we discuss findings pertaining to the requirement of de novo protein synthesis to memory formation, how local dendritic and axonal translation is regulated in neurons, and how these can influence memory consolidation. We also highlight the importance of eIF2α-dependent translation initiation to synaptic plasticity and memory formation. Finally, we contextualize how aberrant phosphorylation of eIF2α contributes to Alzheimer's disease (AD) pathology and how preventing disruption of eIF2-dependent translation may be a therapeutic avenue for preventing and/or restoring memory loss in AD.
Keywords: Alzheimer’s disease; EIF2α; Integrated stress response; MRNA translation; Memory consolidation; Protein synthesis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

References
-
- Tonegawa S, Morrissey MD, Kitamura T, The role of engram cells in the systems consolidation of memory, Nat Rev Neurosci 19(8) (2018) 485–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

