A Computational Study of the Electrophysiological Substrate in Patients Suffering From Atrial Fibrillation
- PMID: 34305637
- PMCID: PMC8297688
- DOI: 10.3389/fphys.2021.673612
A Computational Study of the Electrophysiological Substrate in Patients Suffering From Atrial Fibrillation
Abstract
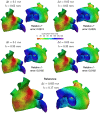
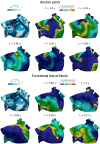
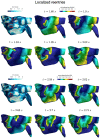
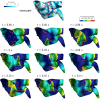
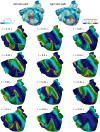
In the context of cardiac electrophysiology, we propose a novel computational approach to highlight and explain the long-debated mechanisms behind atrial fibrillation (AF) and to reliably numerically predict its induction and sustainment. A key role is played, in this respect, by a new way of setting a parametrization of electrophysiological mathematical models based on conduction velocities; these latter are estimated from high-density mapping data, which provide a detailed characterization of patients' electrophysiological substrate during sinus rhythm. We integrate numerically approximated conduction velocities into a mathematical model consisting of a coupled system of partial and ordinary differential equations, formed by the monodomain equation and the Courtemanche-Ramirez-Nattel model. Our new model parametrization is then adopted to predict the formation and self-sustainment of localized reentries characterizing atrial fibrillation, by numerically simulating the onset of ectopic beats from the pulmonary veins. We investigate the paroxysmal and the persistent form of AF starting from electro-anatomical maps of two patients. The model's response to stimulation shows how substrate characteristics play a key role in inducing and sustaining these arrhythmias. Localized reentries are less frequent and less stable in case of paroxysmal AF, while they tend to anchor themselves in areas affected by severe slow conduction in case of persistent AF.
Keywords: arrhythmia; atrial fibrillation; cardiac electrophysiology; mathematical models; numerical simulation.
Copyright © 2021 Pagani, Dede', Frontera, Salvador, Limite, Manzoni, Lipartiti, Tsitsinakis, Hadjis, Della Bella and Quarteroni.
Conflict of interest statement
AF and PD have received consultant fees from Boston Scientific. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Arndt D., Bangerth W., Blais B., Clevenger T. C., Fehling M., Grayver A. V., et al. . (2020a). The deal.II library, version 9.2. J. Numerical Math. 28, 131–146. 10.1515/jnma-2020-0043 - DOI
-
- Arndt D., Bangerth W., Davydov D., Heister T., Heltai L., Kronbichler M., et al. . (2020b). The deal.II finite element library: design, features, and insights. Comput. Math. Appl. 81, 407–422. 10.1016/j.camwa.2020.02.022 - DOI
-
- Aronis K. N., Ali R. L., Prakosa A., Ashikaga H., Berger R. D., Hakim J. B., et al. . (2020). Accurate conduction velocity maps and their association with scar distribution on magnetic resonance imaging in patients with postinfarction ventricular tachycardias. Circ. Arrhythm. Electrophysiol. 13, e007792. 10.1161/CIRCEP.119.007792 - DOI - PMC - PubMed
-
- Boyle P. M., Hakim J. B., Zahid S., Franceschi W. H., Murphy M. J., Vigmond E. J., et al. . (2018). Comparing reentrant drivers predicted by image-based computational modeling and mapped by electrocardiographic imaging in persistent atrial fibrillation. Front. Physiol. 9:414. 10.3389/fphys.2018.00414 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

