Safety of Semaglutide
- PMID: 34305810
- PMCID: PMC8294388
- DOI: 10.3389/fendo.2021.645563
Safety of Semaglutide
Erratum in
-
Corrigendum: Safety of Semaglutide.Front Endocrinol (Lausanne). 2021 Nov 10;12:786732. doi: 10.3389/fendo.2021.786732. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34858353 Free PMC article.
Abstract
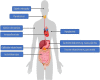
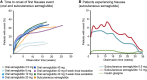
The glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide is the most recently approved agent of this drug class, and the only GLP-1RA currently available as both subcutaneous and oral formulation. While GLP-1RAs effectively improve glycemic control and cause weight loss, potential safety concerns have arisen over the years. For semaglutide, such concerns have been addressed in the extensive phase 3 registration trials including cardiovascular outcome trials for both subcutaneous (SUSTAIN: Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) and oral (PIONEER: Peptide InnOvatioN for the Early diabEtes tReatment) semaglutide and are being studied in further trials and registries, including real world data studies. In the current review we discuss the occurrence of adverse events associated with semaglutide focusing on hypoglycemia, gastrointestinal side effects, pancreatic safety (pancreatitis and pancreatic cancer), thyroid cancer, gallbladder events, cardiovascular aspects, acute kidney injury, diabetic retinopathy (DRP) complications and injection-site and allergic reactions and where available, we highlight potential underlying mechanisms. Furthermore, we discuss whether effects are specific for semaglutide or a class effect. We conclude that semaglutide induces mostly mild-to-moderate and transient gastrointestinal disturbances and increases the risk of biliary disease (cholelithiasis). No unexpected safety issues have arisen to date, and the established safety profile for semaglutide is similar to that of other GLP-1RAs where definitive conclusions for pancreatic and thyroid cancer cannot be drawn at this point due to low incidence of these conditions. Due to its potent glucose-lowering effect, patients at risk for deterioration of existing DRP should be carefully monitored if treated with semaglutide, particularly if also treated with insulin. Given the beneficial metabolic and cardiovascular actions of semaglutide, and the low risk for severe adverse events, semaglutide has an overall favorable risk/benefit profile for patient with type 2 diabetes.
Keywords: glucagon-like peptide-1 receptor agonist (GLP-1RA); oral; safety; semaglutide; subcutaneous; type 2 diabetes.
Copyright © 2021 Smits and Van Raalte.
Conflict of interest statement
DVR has acted as a consultant and received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk and Sanofi and has received research operating funds from the Boehringer Ingelheim–Eli Lilly Diabetes Alliance, MSD, AstraZeneca and Novo Nordisk. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this article received funding from Novo Nordisk. The funder had the following involvement in the article: medical writing support.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas 2019 (2019). Available at: https://www.diabetesatlas.org/data/en/region/3/eur.html. - PubMed
-
- Hussein H, Zaccardi F, Khunti K, Davies MJ, Patsko E, Dhalwani NN, et al. . Efficacy and Tolerability of Sodium-Glucose Co-Transporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Systematic Review and Network Meta-Analysis. Diabetes Obes Metab (2020) 22:1035–46. doi: 10.1111/dom.14008 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

