Prediction of Minocycline Activity in the Gut From a Pig Preclinical Model Using a Pharmacokinetic -Pharmacodynamic Approach
- PMID: 34305836
- PMCID: PMC8299485
- DOI: 10.3389/fmicb.2021.671376
Prediction of Minocycline Activity in the Gut From a Pig Preclinical Model Using a Pharmacokinetic -Pharmacodynamic Approach
Abstract
The increase of multidrug-resistant (MDR) bacteria has renewed interest in old antibiotics, such as minocycline, that can be active against various MDR Gram-negative pathogens. The elimination of minocycline by both kidneys and liver makes it suitable for impaired renal function patients. However, the drawback is the possible elimination of a high amount of drug in the intestines, with potential impact on the digestive microbiota during treatment. This study aimed to predict the potential activity of minocycline against Enterobacterales in the gut after parenteral administration, by combining in vivo and in vitro studies. Total minocycline concentrations were determined by UPLC-UV in the plasma and intestinal content of piglets following intravenous administration. In parallel, the in vitro activity of minocycline was assessed against two Escherichia coli strains in sterilized intestinal contents, and compared to activity in a standard broth. We found that minocycline concentrations were 6-39 times higher in intestinal contents than plasma. Furthermore, minocycline was 5- to 245-fold less active in large intestine content than in a standard broth. Using this PK-PD approach, we propose a preclinical pig model describing the link between systemic and gut exposure to minocycline, and exploring its activity against intestinal Enterobacterales by taking into account the impact of intestinal contents.
Keywords: antibiotic; antimicrobial resistance; binding; commensal flora; digestive concentrations; intestinal contents; microbiota; pig model.
Copyright © 2021 Vallé, Roques, Bousquet-Mélou, Dahlhaus, Ramon-Portugal, Dupouy, Bibbal and Ferran.
Conflict of interest statement
QV is a Virbac employee, however, Virbac was not involved in the experiment design, data analysis or manuscript writing. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


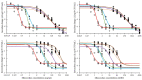
 ), SIC jejunum (
), SIC jejunum ( ), SIC ileum (
), SIC ileum ( ), SIC cecum (
), SIC cecum ( ), SIC colon (
), SIC colon ( ), and SIC feces (
), and SIC feces ( ).
).References
-
- Aranzana-Climent V., Buyck J. M., Smani Y., Pachón-Diaz J., Marchand S., Couet W., et al. (2020). Semi-mechanistic PK/PD modelling of combined polymyxin B and minocycline against a polymyxin-resistant strain of Acinetobacter baumannii. Clin. Microbiol. Infect. 20:S1198743X20300422. 10.1016/j.cmi.2020.01.017 - DOI - PubMed
LinkOut - more resources
Full Text Sources

