The Role of Regulatory T Cells in Epicutaneous Immunotherapy for Food Allergy
- PMID: 34305893
- PMCID: PMC8297384
- DOI: 10.3389/fimmu.2021.660974
The Role of Regulatory T Cells in Epicutaneous Immunotherapy for Food Allergy
Abstract
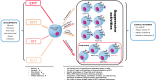
In recent decades, a rapid increase in the prevalence of food allergies has led to extensive research on novel treatment strategies and their mechanisms. Mouse models have provided preliminary insights into the mechanism of epicutaneous immunotherapy (EPIT)-induced immune tolerance. In EPIT, antigen applied on the skin surface can be captured, processed, and presented in the lymph nodes (LNs) by Antigen-presenting cells (APCs). In the LNs, induction of regulatory T cells (Treg cells) requires both direct contact during antigen presentation and indirect mechanisms such as cytokines. Foxp3+CD62L+ Treg cells can exhibit the characteristics of hypomethylation of Foxp3 TSDR and Foxp3-LAP+ Treg cells, which increase the expression of surface tissue-specific homing molecules to exert further sustained systemic immune tolerance. Studies have shown that EPIT is a potential treatment for food allergies and can effectively induce immune tolerance, but its mechanism needs further exploration. Here, we review Treg cells' role in immune tolerance induced by EPIT and provide a theoretical basis for future research directions, such as the mechanism of EPIT and the development of more effective EPIT treatments.
Keywords: allergen-specific immunotherapy (AIT); epicutaneous immunotherapy (EPIT); food allergy; immune tolerance; regulatory T cell (Treg cell).
Copyright © 2021 Liu, Liu, Wang, Mou and Che.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

