From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape
- PMID: 34305909
- PMCID: PMC8293291
- DOI: 10.3389/fimmu.2021.679344
From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape
Abstract
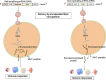
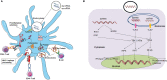
Recently, mRNA vaccines have become a significant type of therapeutic and have created new fields in the biopharmaceutical industry. mRNA vaccines are promising next-generation vaccines that have introduced a new age in vaccinology. The recent approval of two COVID-19 mRNA vaccines (mRNA-1273 and BNT162b2) has accelerated mRNA vaccine technology and boosted the pharmaceutical and biotechnology industry. These mRNA vaccines will help to tackle COVID-19 pandemic through immunization, offering considerable hope for future mRNA vaccines. Human trials with data both from mRNA cancer vaccines and mRNA infectious disease vaccines have provided encouraging results, inspiring the pharmaceutical and biotechnology industries to focus on this area of research. In this article, we discuss current mRNA vaccines broadly in two parts. In the first part, mRNA vaccines in general and COVID-19 mRNA vaccines are discussed. We presented the mRNA vaccine structure in general, the different delivery systems, the immune response, and the recent clinical trials for mRNA vaccines (both for cancer mRNA vaccines and different infectious diseases mRNA vaccines). In the second part, different COVID-19 mRNA vaccines are explained. Finally, we illustrated a snapshot of the different leading mRNA vaccine developers, challenges, and future prospects of mRNA vaccines.
Keywords: BNT162b2; COVID-19; mRNA vaccine developers; mRNA vaccines; mRNA-1273.
Copyright © 2021 Chakraborty, Sharma, Bhattacharya and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

