Evaluation of intracellular survival of Campylobacter fetus subsp. fetus in bovine endometrial cells by qPCR
- PMID: 34306105
- PMCID: PMC8294819
- DOI: 10.22099/ijvr.2021.38693.5632
Evaluation of intracellular survival of Campylobacter fetus subsp. fetus in bovine endometrial cells by qPCR
Abstract
Background: Campylobacter fetus subsp. fetus is the causal agent of sporadic abortion and infertility in bovines that produces economic losses in livestock.
Aims: This study evaluates the capability of C. fetus subsp. fetus to invade and survive in bovine endometrial epithelial cells and attempts to describe a pathogenic mechanism of this microorganism.
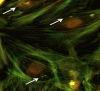
Methods: Primary culture of bovine endometrial epithelial cells was challenged with C. fetus subsp. fetus. Intracellular bacteria, represented by the number of genomic copies (g.c.) were quantified at 0, 2, 4, 10, and 24 hours post-infection (h.p.i.), by quantitative polymerase chain reaction (qPCR). The presence of intracellular bacteria was evaluated by immunofluorescence and immunohistochemistry.
Results: The results showed that only viable C. fetus subsp. fetus could invade endometrial cells. The g.c. number in assays with viable C. fetus subsp. fetus reached an average value of 656 g.c., remained constant until 4 h.p.i., then decreased to 100 g.c, at 24 h.p.i. In assays with non-viable microorganisms, the average value of g.c. was less than 1 g.c. and never changed. The intracellular presence of this bacteria was confirmed at 2 h.p.i. by immunofluorescence and immunohistochemistry.
Conclusion: The results suggest that only C. fetus subsp. fetus viable can invade bovine endometrial epithelial cells but will not replicate in them, indicating that the endometrial cells do not represent a replication niche for this pathogen. Nonetheless, this invasion capability suggests that this type of cell could be employed by the pathogen to spread to other tissues.
Keywords: Bovine endometrial cells; Campylobacter fetus; Intracellular survival.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2021 Mar;70(1):99-106. doi: 10.33073/pjm-2021-009. Epub 2021 Mar 19. Pol J Microbiol. 2021. PMID: 33815531 Free PMC article.
-
Campylobacter fetus is Internalized by Bovine Endometrial Epithelial Cells.Pol J Microbiol. 2019;68(2):217-224. doi: 10.33073/pjm-2019-022. Pol J Microbiol. 2019. PMID: 31250592 Free PMC article.
-
Genomic analysis of Campylobacter fetus subspecies: identification of candidate virulence determinants and diagnostic assay targets.BMC Microbiol. 2009 May 8;9:86. doi: 10.1186/1471-2180-9-86. BMC Microbiol. 2009. PMID: 19422718 Free PMC article.
-
[Occurrence and significance of different Campylobacter types in domestic animals in Hungary].Dtsch Tierarztl Wochenschr. 1990 Aug;97(8):317-21. Dtsch Tierarztl Wochenschr. 1990. PMID: 2209454 Review. German.
-
Campylobacter-Associated Diseases in Animals.Annu Rev Anim Biosci. 2017 Feb 8;5:21-42. doi: 10.1146/annurev-animal-022516-022826. Epub 2016 Nov 9. Annu Rev Anim Biosci. 2017. PMID: 27860495 Review.
Cited by
-
DNA extraction leads to bias in bacterial quantification by qPCR.Appl Microbiol Biotechnol. 2022 Dec;106(24):7993-8006. doi: 10.1007/s00253-022-12276-4. Epub 2022 Nov 14. Appl Microbiol Biotechnol. 2022. PMID: 36374332 Free PMC article. Review.
References
-
- Alge CS, Hauck SM, Priglinger SG, Kampik A, Ueffing M. Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome Res. 2006;5:862–878. - PubMed
-
- Baker NT, Graham LL. Campylobacter fetus translocation across Caco-2 cell monolayers. Microb. Pathog. 2010;49:260–272. - PubMed
-
- Boumart Z, Velge P, Wiedemann A. Multiple invasion mechanisms and different intracellular behaviors: a new vision of Salmonella–host cell interaction. FEMS Microbiol. Lett. 2014;361:1–7. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
