ZFAS1 knockdown inhibits fibroblast-like synoviocyte proliferation, migration, invasion and inflammation, and promotes apoptosis via miR-3926/FSTL1 in rheumatoid arthritis
- PMID: 34306188
- PMCID: PMC8281480
- DOI: 10.3892/etm.2021.10346
ZFAS1 knockdown inhibits fibroblast-like synoviocyte proliferation, migration, invasion and inflammation, and promotes apoptosis via miR-3926/FSTL1 in rheumatoid arthritis
Abstract
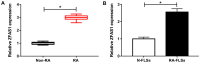
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by joint disorders. Long non-coding RNA zinc finger antisense 1 (ZFAS1) is aberrantly expressed in numerous human diseases, including RA. The present study aimed to investigate the functions and underlying mechanisms of ZFAS1 in RA. Reverse transcription-quantitative PCR was performed to determine the expression levels of ZFAS1, microRNA (miR)-3926 and follistatin-like protein 1 (FSTL1). MTT assay, flow cytometric analysis and Transwell assay were performed to examine the proliferation, apoptosis, migration and invasion of fibroblast-like synoviocytes (FLSs), respectively. Western blotting was employed to measure the protein expression levels of cleaved caspase-3, interleukin (IL)-6, IL-1β, tumor necrosis factor-α and FSTL1. Dual-luciferase reporter assay was performed to verify the interaction between miR-3926 and ZFAS1 or FSTL1. The results demonstrated that ZFAS1 and FSTL1 were upregulated, and miR-3926 was downregulated in RA synovial tissues and RA-FLSs. ZFAS1 knockdown suppressed cell proliferation, migration, invasion and inflammatory cytokine production, and induced apoptosis in RA-FLSs. ZFAS1 acted as a sponge for miR-3926, and ZFAS1 overexpression abolished the impact of miR-3926 on the development of RA-FLSs. FSTL1 was a direct target of miR-3926, and the effect of FSTL1 knockdown on the progression of RA-FLSs was rescued by miR-3926 inhibition. Furthermore, ZFAS1 regulated FSTL1 expression levels via sponging miR-3926 in RA-FLSs. In conclusion, ZFAS1 knockdown inhibited RA-FLS proliferation, migration, invasion and inflammatory cytokine production, and induced apoptosis in RA via the miR-3926/FSTL1 axis.
Keywords: fibroblast-like synoviocytes; follistatin-like protein 1; microRNA-3926; rheumatoid arthritis; zinc finger antisense 1.
Copyright © 2020, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
