Assessment of the control measures of the category A diseases of Animal Health Law: Classical Swine Fever
- PMID: 34306220
- PMCID: PMC8294054
- DOI: 10.2903/j.efsa.2021.6707
Assessment of the control measures of the category A diseases of Animal Health Law: Classical Swine Fever
Abstract
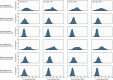
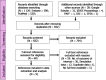
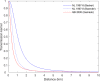
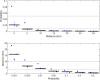
EFSA received a mandate from the European Commission to assess the effectiveness of some of the control measures against diseases included in the Category A list according to Regulation (EU) 2016/429 on transmissible animal diseases ('Animal Health Law'). This opinion belongs to a series of opinions where these control measures will be assessed, with this opinion covering the assessment of control measures for Classical swine fever (CSF). In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of: (i) clinical and laboratory sampling procedures, (ii) monitoring period and (iii) the minimum radii of the protection and surveillance zones, and the minimum length of time the measures should be applied in these zones. The general methodology used for this series of opinions has been published elsewhere; nonetheless, details of the model used for answering these questions are presented in this opinion as well as the transmission kernels used for the assessment of the minimum radius of the protection and surveillance zones. Several scenarios for which these control measures had to be assessed were designed and agreed prior to the start of the assessment. Here, several recommendations are given on how to increase the effectiveness of some of the sampling procedures. Based on the average length of the period between virus introduction and the reporting of a CSF suspicion, the monitoring period was assessed as non-effective. In a similar way, it was recommended that the length of the measures in the protection and surveillance zones were increased from 15 to 25 days in the protection zone and from 30 to 40 days in the surveillance zone. Finally, the analysis of existing Kernels for CSF suggested that the radius of the protection and the surveillance zones comprise 99% of the infections from an affected establishment if transmission occurred. Recommendations provided for each of the scenarios assessed aim to support the European Commission in the drafting of further pieces of legislation, as well as for plausible ad hoc requests in relation to CSF.
Keywords: CSF; disease control; intervention; monitoring period; protection zone; sampling procedures; surveillance zone.
© 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
Similar articles
-
Scientific Opinion on the assessment of the control measures for category A diseases of Animal Health Law: Foot and Mouth Disease.EFSA J. 2021 Jun 8;19(6):e06632. doi: 10.2903/j.efsa.2021.6632. eCollection 2021 Jun. EFSA J. 2021. PMID: 34136003 Free PMC article.
-
Assessment of the control measures for category A diseases of Animal Health Law: Lumpy Skin Disease.EFSA J. 2022 Jan 24;20(1):e07121. doi: 10.2903/j.efsa.2022.7121. eCollection 2022 Jan. EFSA J. 2022. PMID: 35106095 Free PMC article.
-
Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: African Swine Fever.EFSA J. 2021 Jan 31;19(1):e06402. doi: 10.2903/j.efsa.2021.6402. eCollection 2021 Jan. EFSA J. 2021. PMID: 33552298 Free PMC article.
-
Classical swine fever in Europe--the current situation.Berl Munch Tierarztl Wochenschr. 2013 Nov-Dec;126(11-12):468-75. Berl Munch Tierarztl Wochenschr. 2013. PMID: 24511821 Review.
-
Laboratory experience during the classical swine fever virus epizootic in the Netherlands in 1997-1998.Vet Microbiol. 2000 Apr 13;73(2-3):197-208. doi: 10.1016/s0378-1135(00)00145-0. Vet Microbiol. 2000. PMID: 10785328 Review.
Cited by
-
The regulation of cell homeostasis and antiviral innate immunity by autophagy during classical swine fever virus infection.Emerg Microbes Infect. 2023 Dec;12(1):2164217. doi: 10.1080/22221751.2022.2164217. Emerg Microbes Infect. 2023. PMID: 36583373 Free PMC article.
-
Postmortem Sampling in Piglet Populations: Unveiling Specimens Accuracy for Porcine Reproductive and Respiratory Syndrome Detection.Pathogens. 2024 Aug 2;13(8):649. doi: 10.3390/pathogens13080649. Pathogens. 2024. PMID: 39204249 Free PMC article.
-
Active Participatory Regional Surveillance for Notifiable Swine Pathogens.Animals (Basel). 2024 Jan 11;14(2):233. doi: 10.3390/ani14020233. Animals (Basel). 2024. PMID: 38254402 Free PMC article.
-
A Quadruplex RT-qPCR for the Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, and Porcine Pseudorabies Virus.Animals (Basel). 2024 Dec 9;14(23):3551. doi: 10.3390/ani14233551. Animals (Basel). 2024. PMID: 39682516 Free PMC article.
-
Eleven Years of Health Monitoring in Wild Boars (Sus scrofa) in the Emilia-Romagna Region (Italy).Animals (Basel). 2023 May 31;13(11):1832. doi: 10.3390/ani13111832. Animals (Basel). 2023. PMID: 37889705 Free PMC article.
References
-
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198.
-
- Blome S, Gabriel C, Schmeiser S, Meyer D, Meindl‐Böhmer A, Koenen F and Beer M, 2014. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Veterinary Microbiology, 169, 8–17. - PubMed
-
- David D, Edri N, Yakobson B, Bombarov V, King R, Davidson I, Pozzi P, Hadani Y, Bellaiche M and Schmeiser S, 2011. Emergence of classical swine fever virus in Israel in 2009. The Veterinary Journal, 190, e146–e149. - PubMed
