The Effectiveness of 3 Combined Therapeutic Regimens in Egyptian Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease: A Randomized Double-Blind Prospective Pilot Study
- PMID: 34306265
- PMCID: PMC8296082
- DOI: 10.1016/j.curtheres.2021.100625
The Effectiveness of 3 Combined Therapeutic Regimens in Egyptian Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease: A Randomized Double-Blind Prospective Pilot Study
Abstract
Background: There are differences of opinion about both the most effective combined therapeutic strategy and the clinical benefit of inhaled corticosteroids in nonasthmatic patients with chronic obstructive pulmonary disease. Furthermore, many inflammatory cytokines are reportedly correlated with severity of the disease.
Objectives: To compare the effectiveness of long acting β-agonist + long-acting muscarinic antagonist (LABA + LAMA) versus LABA + inhaled corticosteroid and LAMA + inhaled corticosteroid in nonasthmatic patients with moderate-to-severe chronic obstructive pulmonary disease. To assess the changes that occurred in plasma concentrations of tumor necrosis factor α, fibrinogen, and interleukin 6, and correlate these with disease activity.
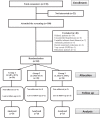
Methods: In this pilot study, 45 nonasthmatic patients with moderate to severe chronic obstructive pulmonary disease were randomized into 3 groups with 15 patients in each group. Group I (LABA + inhaled corticosteroid) received formoterol/budesonide, group II (LAMA + inhaled corticosteroid) received tiotropium/budesonide and group III (LABA + LAMA) received formoterol/tiotropium for 12 weeks. Patients were assessed initially and then at 4 and 12 weeks by measuring the changes that occurred in forced expiratory volume in 1 second as a percent of predicted and in the modified Medical Research Council dyspnea scale. Plasma concentrations of tumor necrosis factor α, fibrinogen, and interleukin 6 were simultaneously measured.
Results: The 3 study groups were statistically similar with respect to their demographic data and disease characteristics. All therapeutic options produced an improvement in forced expiratory volume in 1 second as a percent of predicted and in the modified Medical Research Council dyspnea scale as well as a reduction in plasma concentrations of the inflammatory markers. The effects produced by the three therapeutic combinations on forced expiratory volume in 1 second as a percent of predicted, plasma tumor necrosis factor α, interleukin 6, and fibrinogen concentrations were statistically similar after 4 and 12 weeks (4 weeks after treatment: P = 0.358, P = 0.284, P = 0.155, and P = 0.155, respectively, and 12 weeks after treatment: P = 0.710, P = 0.773, P = 0.240, and P = 0.076, respectively).
Conclusions: In nonasthmatic patients with moderate to severe chronic obstructive pulmonary disease, the 3 therapeutic combinations showed similar effectiveness. The results of this pilot study also suggest that inflammatory markers can be used to track disease activity. Clinicaltrials.gov identifier: NCT04520230. (Curr Ther Res Clin Exp. 2021; 82:XXX-XXX).
Keywords: COPD; Fibrinogen; ICS; LABA; LAMA; TNF-ɑ.
© 2021 The Authors.
Conflict of interest statement
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease [GOLD]. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. (2018). Available at: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev... (accessed November 20, 2018).
Associated data
LinkOut - more resources
Full Text Sources
Medical