PLAC8 gene knockout increases the radio-sensitivity of xenograft tumors in nude mice with nasopharyngeal carcinoma by promoting apoptosis
- PMID: 34306339
- PMCID: PMC8290649
PLAC8 gene knockout increases the radio-sensitivity of xenograft tumors in nude mice with nasopharyngeal carcinoma by promoting apoptosis
Abstract
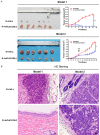
In vitro cell experiments showed that knocking out the placenta-specific protein 8 (PLAC8) gene significantly increased the sensitivity of tumor cells to radiation. This study used two nude mouse models of nasopharyngeal carcinoma (NPC) to investigate the radio-sensitization and molecular mechanism of PLAC8 knockout in vivo. The expression of PLAC8 in 120 NPC tissues and 30 nasopharyngitis (NPG) tissues was detected by immunohistochemistry (IHC) to analyze the relationship between PLAC8 and neck lymph node metastasis and prognosis in NPC patients. The mRNA expression level of PLAC8 in several NPC cell lines was detected by semi-quantitative RT-PCR. The PLAC8 gene was knocked out in CNE-2 cells using CRISPR/Cas9. The effect of PLAC8 gene knockout on the radiotherapy sensitivity of NPC cells was analyzed by establishing model 1 and model 2 tumor-bearing nude mouse models with two different irradiation methods. The expression of γH2AX, Bax, Bcl-2, Caspase-3 and cleaved Caspase-3 was detected by immunofluorescence (IF), IHC and western blot analysis. PLAC8 expression was significantly increased in NPC tissue samples and NPC cell lines compared with NPG tissue samples and normal cell lines (P<0.01). PLAC8 upregulation was associated with lymph node metastasis and a poor prognosis in patients with NPC (P<0.01). Both animal models showed that radiotherapy after PLAC8 knockout significantly slowed tumor growth and reduced tumor volume, with tumor inhibition rates of 100% and 66.04%, respectively. In model 2, PLAC8 knockout with radiotherapy increased the expressions of γH2AX, Bax, Caspase-3 and cleaved Caspase-3 but decreased the expression of Bcl-2 (P<0.01). In model 1, there was no tumor formation at the site where the cancer cells were injected. The expression levels of γH2AX, Bax, Caspase-3 and cleaved Caspase-3 in skin tissues taken at the injection site were lower than those in NPC tissues treated with radiotherapy, while the expression level of Bcl-2 was higher (P<0.01). PLAC8 expression is closely related to neck metastasis and the prognosis of NPC. PLAC8 gene knockout significantly increases the radio-sensitivity of NPC cells in vivo by promoting apoptosis, which is an effective strategy for the radiotherapy sensitization of NPC.
Keywords: NPC; PLAC8; apoptosis; radio-sensitivity; subcutaneously transplanted tumor model.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures





References
-
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. - PubMed
-
- Carioli G, Negri E, Kawakita D, Garavello W, La Vecchia C, Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int J Cancer. 2017;140:2256–2264. - PubMed
-
- Tian Y, Yan M, Zheng J, Li R, Lin J, Xu A, Liang Y, Zheng R, Yuan Y. miR-483-5p decreases the radiosensitivity of nasopharyngeal carcinoma cells by targeting DAPK1. Lab Invest. 2019;99:602–611. - PubMed
-
- Wang C, Su Z, Hou H, Li D, Pan Z, Tian W, Mo C. Inhibition of anaphase-promoting complex by silence APC/C(Cdh1) to enhance radiosensitivity of nasopharyngeal carcinoma cells. J Cell Biochem. 2017;118:3150–3157. - PubMed
-
- Lan R, Huang F, Zhong G, Chen R, Wang Z, Chen J, Fu L, Hong J, Zhang L. Effects of CKMT1 on radiosensitivity of nasopharyngeal carcinoma cells. Int J Radiat Biol. 2019;95:597–606. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
