Nickel-Catalyzed Decarbonylative Synthesis of Fluoroalkyl Thioethers
- PMID: 34306801
- PMCID: PMC8294461
- DOI: 10.1021/acscatal.0c02950
Nickel-Catalyzed Decarbonylative Synthesis of Fluoroalkyl Thioethers
Abstract
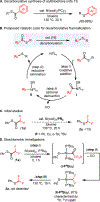
This report describes the development of a nickel-catalyzed decarbonylative reaction for the synthesis of fluoroalkyl thioethers (RFSR) from the corresponding thioesters. Readily available, inexpensive, and stable fluoroalkyl carboxylic acids (RFCO2H) serve as the fluoroalkyl (RF) source in this transformation. Stoichiometric organometallic studies reveal that RF-S bond-forming reductive elimination is a challenging step in the catalytic cycle. This led to the identification of diphenylphosphinoferrocene as the optimal ligand for this transformation. Ultimately, this method was applied to the construction of diverse fluoroalkyl thioethers (RFSR), with R = both aryl and alkyl.
Keywords: Nickel-catalysis; decarbonylation; fluoroalkyl carboxylic acids; fluoroalkylation; thioether synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Purser S; Moore PR; Swallow S; Gouverneur V Fluorine in medicinal chemistry. Chem. Soc. Rev 2008, 37, 320–330. - PubMed
- Hagmann WK The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem 2008, 51, 4359–4369. - PubMed
- Meanwell NA Synopsis of Some Recent Tactical Applications of Bioisoteres in Drug Design. J. Med. Chem 2011, 54, 2529–2591. - PubMed
- Leroux F; Jeschke P; Schlosser M α-Fluorinated Ethers, Thioethers, and Amines: Anomerically Biased species. Chem. Rev 2005, 105, 827–856. - PubMed
- Zafrani Y; Yeffet D; Sod-Moriah G; Berliner A; Amier D; Marciano D; Gershonov E; Saphier S Difluoromethyl Bioiostere: Examining the “Lipophilic Hydrogen Bond Donor” Concept. J. Med. Chem 2017, 60, 797–804. - PubMed
-
- Röschenthaler GV; Kazakova O Chemistry of α-Fluorinated Ethers- and Thioethers-Containing Heterocycles. In Fluorine in Heterocyclic Chemistry Nenajdenko V Eds.; Springer: Moscow, 2014.
- Kaiser F; Grob S; Langewald J; Narine A Insecticidal Active Mixtures Comprising Arylquinazolinone Compounds WO 2013/030262, August 13, 2012.
-
- Xiong H; Pannecoucke X; Besset T Recent Advances in the Synthesis of SCF2H- and SCF2FG- Containing Molecules. Chem. Eur. J 2016, 16734–16749. - PubMed
-
- Hine J; Porter JJ Methylene Derivatives as Intermediates in Polar Reactions. J. Am. Chem. Soc 1957, 79, 5493–5496.
- Langlois BR Improvement of the Synthesis of Aryl Difluoromethyl Ethers and Thioethers by Using a Solid–Liquid Phase–Transfer Technique. J. Fluorine Chem 1988, 41, 247–261.
- Mehta VP; Greaney MF S-, N-, and Se-Difluoromethylation Using Sodium Chlorodifluoroacetate. Org. Lett 2013, 15, 5036–5039. - PubMed
-
- Zhang W; Wang F; Hu J N-Tosyl-S-Difluoromethyl-S-phenylsulfoximine: A New Difluoromethylation Reagent for S-, N- and C-Nucleophiles. Org. Lett 2009, 11, 2109–2112. - PubMed
- Zafrani Y; Sod-Moriah G; Segall Y Diethyl Bromodifluoromethylphosphonate: A Highly Efficient and Environmentally Benign Difluorocarbene Precursor. Tetrahedron 2009, 65, 5278–5283.
- Fier PS; Hartwig JF Synthesis of Difluoromethyl Ethers with Difluoromethyltriflate. Angew. Chem. Int. Ed 2013, 52, 2092–2095. - PMC - PubMed
- Thomoson CS; Dolbier WR Jr. Use of Fluoroform as a Source of Difluorocarbene in the Synthesis of Difluoromethoxy- and Difluorothiomethoxyarenes. J. Org. Chem 2013, 78, 8904–8908. - PubMed
- Deng X-Y; Lin J-H; Zheng J; Xiao J-C Difluoromethylation and gem-Difluorocyclopropenation with Difluorocarbene Generated by Decarboxylation. Chem. Commun 2015, 51, 8805–8808. - PubMed
- Orsi DL; Easley BJ; Lick AM; Altman RA Base Catalysis Enables Access to α, α-Difluoroalkylthioethers. Org. Lett 2017, 19, 1570–1573. - PMC - PubMed
- Straathof NJW; Tegelbeckers BJP; Hessel V; Wang X; Noël T A Mild and Fast Photocatalyic Trifluoromethylation of Thiols in Batch and Continuous-Flow. Chem. Sci 2014, 5, 4768–4773.
- Bottecchia C; Wei X-J; Kuijpers KPL; Hessel V; Noël T Visible Light-Induced Trifluoromethylation and Pefluoroalkylation of Cysteine Residues in Batch and Continuous Flow. J. Org. Chem 2016. 81, 7301–7307. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
