Targeting Glioblastoma Stem Cells: A Review on Biomarkers, Signal Pathways and Targeted Therapy
- PMID: 34307170
- PMCID: PMC8297686
- DOI: 10.3389/fonc.2021.701291
Targeting Glioblastoma Stem Cells: A Review on Biomarkers, Signal Pathways and Targeted Therapy
Abstract
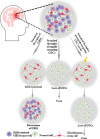
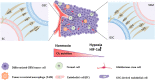
Glioblastoma (GBM) remains the most lethal and common primary brain tumor, even after treatment with multiple therapies, such as surgical resection, chemotherapy, and radiation. Although great advances in medical development and improvements in therapeutic methods of GBM have led to a certain extension of the median survival time of patients, prognosis remains poor. The primary cause of its dismal outcomes is the high rate of tumor recurrence, which is closely related to its resistance to standard therapies. During the last decade, glioblastoma stem cells (GSCs) have been successfully isolated from GBM, and it has been demonstrated that these cells are likely to play an indispensable role in the formation, maintenance, and recurrence of GBM tumors, indicating that GSCs are a crucial target for treatment. Herein, we summarize the current knowledge regarding GSCs, their related signaling pathways, resistance mechanisms, crosstalk linking mechanisms, and microenvironment or niche. Subsequently, we present a framework of targeted therapy for GSCs based on direct strategies, including blockade of the pathways necessary to overcome resistance or prevent their function, promotion of GSC differentiation, virotherapy, and indirect strategies, including targeting the perivascular, hypoxic, and immune niches of the GSCs. In summary, targeting GSCs provides a tremendous opportunity for revolutionary approaches to improve the prognosis and therapy of GBM, despite a variety of challenges.
Keywords: biomarkers; glioblastoma; glioblastoma stem cells; signal pathways; targeted therapy.
Copyright © 2021 Tang, Zuo, Fang, Liu, Qiu, Huang and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Role of Hypoxia and Cancer Stem Cells in Development of Glioblastoma.Cancers (Basel). 2023 May 4;15(9):2613. doi: 10.3390/cancers15092613. Cancers (Basel). 2023. PMID: 37174078 Free PMC article. Review.
-
Targeting Glioblastoma Stem Cells to Overcome Chemoresistance: An Overview of Current Therapeutic Strategies.Biomedicines. 2022 Jun 2;10(6):1308. doi: 10.3390/biomedicines10061308. Biomedicines. 2022. PMID: 35740330 Free PMC article. Review.
-
The Importance of Tumor Stem Cells in Glioblastoma Resistance to Therapy.Int J Mol Sci. 2021 Apr 8;22(8):3863. doi: 10.3390/ijms22083863. Int J Mol Sci. 2021. PMID: 33917954 Free PMC article. Review.
-
The Discovery of RNA Aptamers that Selectively Bind Glioblastoma Stem Cells.Mol Ther Nucleic Acids. 2019 Dec 6;18:99-109. doi: 10.1016/j.omtn.2019.08.015. Epub 2019 Aug 22. Mol Ther Nucleic Acids. 2019. PMID: 31541799 Free PMC article.
-
The hypoxic peri-arteriolar glioma stem cell niche, an integrated concept of five types of niches in human glioblastoma.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):346-354. doi: 10.1016/j.bbcan.2018.04.008. Epub 2018 Apr 21. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29684521 Review.
Cited by
-
Live Organoid Cyclic Imaging.Adv Sci (Weinh). 2024 Apr;11(14):e2309289. doi: 10.1002/advs.202309289. Epub 2024 Feb 7. Adv Sci (Weinh). 2024. PMID: 38326078 Free PMC article.
-
HIF2α Upregulates the Migration Factor ODZ1 under Hypoxia in Glioblastoma Stem Cells.Int J Mol Sci. 2022 Jan 11;23(2):741. doi: 10.3390/ijms23020741. Int J Mol Sci. 2022. PMID: 35054927 Free PMC article.
-
lncRNA Biomarkers of Glioblastoma Multiforme.Biomedicines. 2024 Apr 23;12(5):932. doi: 10.3390/biomedicines12050932. Biomedicines. 2024. PMID: 38790894 Free PMC article. Review.
-
Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics.Sensors (Basel). 2024 Apr 30;24(9):2865. doi: 10.3390/s24092865. Sensors (Basel). 2024. PMID: 38732975 Free PMC article. Review.
-
Immunocompetent murine glioblastoma stem-like cell models exhibiting distinct phenotypes.Neurooncol Adv. 2024 Dec 7;7(1):vdae215. doi: 10.1093/noajnl/vdae215. eCollection 2025 Jan-Dec. Neurooncol Adv. 2024. PMID: 39896074 Free PMC article.
References
-
- Tasaki T, Fujita M, Okuda T, Yoneshige A, Kato A. MET Expressed in Glioma Stem Cells Is a Potent Therapeutic Target for Glioblastoma Multiform. Anticancer Res (2016) 36(7):3571. - PubMed
-
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. . Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro Oncol (2020) 22(8):1073–113. 10.1093/neuonc/noaa106 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

