SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro
- PMID: 34307198
- PMCID: PMC8292147
- DOI: 10.3389/fcimb.2021.701278
SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro
Abstract
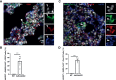
SARS-CoV-2 infection can cause fatal inflammatory lung pathology, including thrombosis and increased pulmonary vascular permeability leading to edema and hemorrhage. In addition to the lung, cytokine storm-induced inflammatory cascade also affects other organs. SARS-CoV-2 infection-related vascular inflammation is characterized by endotheliopathy in the lung and other organs. Whether SARS-CoV-2 causes endotheliopathy by directly infecting endothelial cells is not known and is the focus of the present study. We observed 1) the co-localization of SARS-CoV-2 with the endothelial cell marker CD31 in the lungs of SARS-CoV-2-infected mice expressing hACE2 in the lung by intranasal delivery of adenovirus 5-hACE2 (Ad5-hACE2 mice) and non-human primates at both the protein and RNA levels, and 2) SARS-CoV-2 proteins in endothelial cells by immunogold labeling and electron microscopic analysis. We also detected the co-localization of SARS-CoV-2 with CD31 in autopsied lung tissue obtained from patients who died from severe COVID-19. Comparative analysis of RNA sequencing data of the lungs of infected Ad5-hACE2 and Ad5-empty (control) mice revealed upregulated KRAS signaling pathway, a well-known pathway for cellular activation and dysfunction. Further, we showed that SARS-CoV-2 directly infects mature mouse aortic endothelial cells (AoECs) that were activated by performing an aortic sprouting assay prior to exposure to SARS-CoV-2. This was demonstrated by co-localization of SARS-CoV-2 and CD34 by immunostaining and detection of viral particles in electron microscopic studies. Moreover, the activated AoECs became positive for ACE-2 but not quiescent AoECs. Together, our results indicate that in addition to pneumocytes, SARS-CoV-2 also directly infects mature vascular endothelial cells in vivo and ex vivo, which may contribute to cardiovascular complications in SARS-CoV-2 infection, including multipleorgan failure.
Keywords: SARS-CoV-2; animal models; aorta ring; endothelial cell infection; hACE2.
Copyright © 2021 Liu, Han, Blair, Kenst, Qin, Upcin, Wörsdörfer, Midkiff, Mudd, Belyaeva, Milligan, Rorison, Wagner, Bodem, Dölken, Aktas, Vander Heide, Yin, Kolls, Roy, Rappaport, Ergün and Qin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bhatnagar J., Gary J., Reagan-Steiner S., Estetter L. B., Tong S., Tao Y., et al. . (2021). Evidence of SARS-CoV-2 Replication and Tropism in the Lungs, Airways and Vascular Endothelium of Patients With Fatal COVID-19: An Autopsy Case-Series. J. Infect. Dis 223, 752–764. 10.1093/infdis/jiab039 - DOI - PMC - PubMed
-
- Blerina Ahmetaj-Shala T. P. P., Baillon L., Swann O. C., Gashaw H., Barclay W. S., Mitchell J. A. (2021). Resistance of Endothelial Cells to SARS-CoV-2 Infection In Vitro . BioRxiv.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

