Co-Immobilized Capillary Enzyme Reactor Based on Beta-Secretase1 and Acetylcholinesterase: A Model for Dual-Ligand Screening
- PMID: 34307303
- PMCID: PMC8295500
- DOI: 10.3389/fchem.2021.708374
Co-Immobilized Capillary Enzyme Reactor Based on Beta-Secretase1 and Acetylcholinesterase: A Model for Dual-Ligand Screening
Abstract
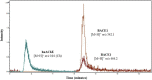
We have developed a dual enzymatic system assay involving liquid chromatography-mass spectrometry (LC-MS) to screen AChE and BACE1 ligands. A fused silica capillary (30 cm × 0.1 mm i.d. × 0.362 mm e.d.) was used as solid support. The co-immobilization procedure encompassed two steps and random immobilization. The resulting huAChE+BACE1-ICER/MS was characterized by using acetylcholine (ACh) and JMV2236 as substrates. The best conditions for the dual enzymatic system assay were evaluated and compared to the conditions of the individual enzymatic system assays. Analysis was performed in series for each enzyme. The kinetic parameters (KMapp) and inhibition assays were evaluated. To validate the system, galantamine and a β-secretase inhibitor were employed as standard inhibitors, which confirmed that the developed screening assay was able to identify reference ligands and to provide quantitative parameters. The combination of these two enzymes in a single on-line system allowed possible multi-target inhibitors to be screened and identified. The innovative huAChE+BACE1-ICER/MS dual enzymatic system reported herein proved to be a reliable tool to identify and to characterize hit ligands for AChE and BACE1 in an enzymatic competitive environment. This innovative system assay involved lower costs; measured the product from enzymatic hydrolysis directly by MS; enabled immediate recovery of the enzymatic activity; showed specificity, selectivity, and sensitivity; and mimicked the cellular process.
Keywords: acetylcholinesterase; beta-secretase1; co-immobilization procedure; dual enzymatic system assay; screening dual- target ligands.
Copyright © 2021 Vilela, Narciso dos Reis and Cardoso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



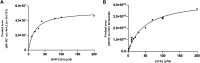

Similar articles
-
Online screening of acetylcholinesterase inhibitors in natural products using monolith-based immobilized capillary enzyme reactors combined with liquid chromatography-mass spectrometry.J Chromatogr A. 2018 Aug 17;1563:135-143. doi: 10.1016/j.chroma.2018.05.069. Epub 2018 May 30. J Chromatogr A. 2018. PMID: 29866504
-
Acetylcholinesterase capillary enzyme reactor for screening and characterization of selective inhibitors.J Pharm Biomed Anal. 2013 Jan 25;73:44-52. doi: 10.1016/j.jpba.2012.01.026. Epub 2012 Feb 18. J Pharm Biomed Anal. 2013. PMID: 22391555
-
A novel on-flow mass spectrometry-based dual enzyme assay.Anal Chim Acta. 2019 Sep 23;1072:81-86. doi: 10.1016/j.aca.2019.04.057. Epub 2019 Apr 26. Anal Chim Acta. 2019. PMID: 31146868
-
An improved immobilized enzyme reactor-mass spectrometry-based label free assay for butyrylcholinesterase ligand screening.Anal Biochem. 2018 May 15;549:53-57. doi: 10.1016/j.ab.2018.03.012. Epub 2018 Mar 14. Anal Biochem. 2018. PMID: 29550345
-
Recent advances in acetylcholinesterase Inhibitors and Reactivators: an update on the patent literature (2012-2015).Expert Opin Ther Pat. 2017 Apr;27(4):455-476. doi: 10.1080/13543776.2017.1272571. Epub 2017 Jan 17. Expert Opin Ther Pat. 2017. PMID: 27967267 Review.
References
-
- Acker M. G., Auld D. S. (2014). Considerations for the Design and Reporting of Enzyme Assays in High-Throughput Screening Applications. Perspect. Sci. 1, 56–73. 10.1016/j.pisc.2013.12.001 - DOI
-
- Basso A., Serban S. (2019). Industrial Applications of Immobilized Enzymes-A Review. Mol. Catal. 479, 110607. 10.1016/j.mcat.2019.110607 - DOI
LinkOut - more resources
Full Text Sources

