Differentially Expressed Circular Non-coding RNAs in Atherosclerotic Aortic Vessels and Their Potential Functions in Endothelial Injury
- PMID: 34307490
- PMCID: PMC8294331
- DOI: 10.3389/fcvm.2021.657544
Differentially Expressed Circular Non-coding RNAs in Atherosclerotic Aortic Vessels and Their Potential Functions in Endothelial Injury
Abstract
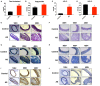
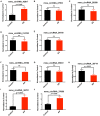
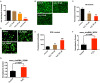
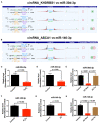
Background: Circular non-coding RNA (circRNA) has a variety of biological functions. However, the expression profile and potential effects of circRNA on atherosclerosis (AS) and vascular endothelial injury have not been fully elucidated. This study aims to identify the differentially expressed circRNAs in atherosclerotic aortic vessels and predict their potential functions in endothelial injury. Method: ApoE-/- mice were fed with high-fat diet for 12 weeks to induce AS. Atherosclerotic plaques were evaluated by H&E and Masson staining and immunohistochemistry; differentially expressed circRNAs were detected by Arraystar Circular RNA Microarray and verified by RT-PCR; the potential target mircoRNAs of circRNAs were predicted by miRanda, Tarbase, Targetscan and their expression changes were verified by RT-PCR; the potential target genes of mircoRNAs were predicted by Targetscan and verified by Western blot; the signaling pathways that they might annotate or regulate and their potential functions in vascular endothelial injury were predicted by gene enrichment analysis. Results: Fifty two circRNAs were up-regulated more than twice and 47 circRNAs were down-regulated more than 1.5 times in AS aortic vessels. Mmmu_circRNA_36781 and 37699 were up-regulated both in AS aortic vessels and H2O2-treated mouse aortic endothelial cells (MAECs). The expression of miR-30d-3p and miR-140-3p, the target microRNA of circRNA_37699 and circRNA_36781, were downregulated both in AS vessels and H2O2-treated MAECs. On the contrary, MKK6 and TP53RK, the potential target gene of miR-140-3p and miR-30d-3p, were upregulated both in AS aortic roots and H2O2-treated MAECs. Besides, gene enrichment analysis showed that MAPK and PI3K-AKT signaling pathway were the most potential signaling pathways regulated by the differentially expressed circRNAs in atherosclerosis. Conclusions: Mmu_circRNA_36781 (circRNA ABCA1) and 37699 (circRNA KHDRBS1) were significantly up-regulated in AS aortic vessels and H2O2-treated MAECs. They have potential regulatory effects on atherosclerosis and vascular endothelial injury by targeting miR-30d-3p-TP53RK and miR-140-3p-MKK6 axis and their downstream signaling pathways.
Keywords: atherosclerosis; ceRNA; circRNA; microRNA; vascular endothelium.
Copyright © 2021 Li, Liu, Sun, Wang, Zhu, Yang, Song, Wang, Wang, Zhao and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, et al. . Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. (2004) 110:571–8. 10.1161/01.CIR.0000136995.83451.1D - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

