Molecular pathways in viral hepatitis-associated liver carcinogenesis: An update
- PMID: 34307543
- PMCID: PMC8283590
- DOI: 10.12998/wjcc.v9.i19.4890
Molecular pathways in viral hepatitis-associated liver carcinogenesis: An update
Abstract
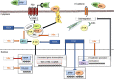
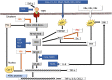
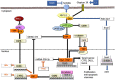
Hepatocellular carcinoma (HCC) is the most common type of cancer among primary malignant tumors of the liver and is a consequential cause of cancer-related deaths worldwide. In recent years, uncovering the molecular mechanisms involved in the development and behavior of this tumor has led to the identification of multiple potential treatment targets. Despite the vast amount of data on this topic, HCC remains a challenging tumor to treat due to its aggressive behavior and complex molecular profile. Therefore, the number of studies aiming to elucidate the mechanisms involved in both carcinogenesis and tumor progression in HCC continues to increase. In this context, the close association of HCC with viral hepatitis has led to numerous studies focusing on the direct or indirect involvement of viruses in the mechanisms contributing to tumor development and behavior. In line with these efforts, this review was undertaken to highlight the current understanding of the molecular mechanisms by which hepatitis B virus (HBV) and hepatitis C virus (HCV) participate in oncogenesis and tumor progression in HCC and summarize new findings. Cumulative evidence indicates that HBV DNA integration promotes genomic instability, resulting in the overexpression of genes related to cancer development, metastasis, and angiogenesis or inactivation of tumor suppressor genes. In addition, genetic variations in HBV itself, especially preS2 deletions, may play a role in malignant transformation. Epigenetic dysregulation caused by both viruses might also contribute to tumor formation and metastasis by modifying the methylation of DNA and histones or altering the expression of microRNAs. Similarly, viral proteins of both HBV and HCV can affect pathways that are important anticancer targets. The effects of these two viruses on the Hippo-Yap-Taz pathway in HCC development and behavior need to be investigated. Additional, comprehensive studies are also needed to determine these viruses' interaction with integrins, farnesoid X, and the apelin system in malignant transformation and tumor progression. Although the relationship of persistent inflammation caused by HBV and HCV hepatitis with carcinogenesis is well defined, further studies are warranted to decipher the relationship among inflammasomes and viruses in carcinogenesis and elucidate the role of virus-microbiota interactions in HCC development and progression.
Keywords: Carcinogenesis; Hepatitis B virus; Hepatitis C virus; Hepatocellular carcinoma; Molecular pathways; Viral hepatitis..
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: No conflict of interest.
Figures

Similar articles
-
Virus associated malignancies: the role of viral hepatitis in hepatocellular carcinoma.Semin Cancer Biol. 2014 Jun;26:78-88. doi: 10.1016/j.semcancer.2014.01.004. Epub 2014 Jan 20. Semin Cancer Biol. 2014. PMID: 24457013 Free PMC article. Review.
-
Hepatocellular carcinoma: The virus or the liver?Liver Int. 2023 Aug;43 Suppl 1:22-30. doi: 10.1111/liv.15253. Epub 2022 Mar 29. Liver Int. 2023. PMID: 35319167 Review.
-
Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma.World J Gastroenterol. 2020 Oct 14;26(38):5759-5783. doi: 10.3748/wjg.v26.i38.5759. World J Gastroenterol. 2020. PMID: 33132633 Free PMC article. Review.
-
Hepatocellular carcinoma and infections with multiple hepatitis viruses.Princess Takamatsu Symp. 1995;25:61-6. Princess Takamatsu Symp. 1995. PMID: 8875610
-
Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias.J Pathol. 2000 Oct;192(2):207-15. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH690>3.0.CO;2-#. J Pathol. 2000. PMID: 11004697
Cited by
-
The Role of Epigallocatechin Gallate (EGCG) in Treatment and Management of Sexually Transmitted Viral Infections.Infect Disord Drug Targets. 2025;25(4):e18715265319110. doi: 10.2174/0118715265319110240916061200. Infect Disord Drug Targets. 2025. PMID: 39482915 Review.
-
Expression of IER3 in hepatocellular carcinoma: clinicopathology, prognosis, and potential regulatory pathways.PeerJ. 2022 Mar 10;10:e12944. doi: 10.7717/peerj.12944. eCollection 2022. PeerJ. 2022. PMID: 35291486 Free PMC article.
-
Signaling Induced by Chronic Viral Hepatitis: Dependence and Consequences.Int J Mol Sci. 2022 Mar 3;23(5):2787. doi: 10.3390/ijms23052787. Int J Mol Sci. 2022. PMID: 35269929 Free PMC article. Review.
-
Clinical Limitations of Tissue Annexin A2 Level as a Predictor of Postoperative Overall Survival in Patients with Hepatocellular Carcinoma.J Clin Med. 2021 Sep 15;10(18):4158. doi: 10.3390/jcm10184158. J Clin Med. 2021. PMID: 34575275 Free PMC article.
-
Myocyte enhancer factor 2D promotes hepatocellular carcinoma through AMOTL2/YAP signaling that inhibited by luteolin.Int J Clin Exp Pathol. 2022 May 15;15(5):206-214. eCollection 2022. Int J Clin Exp Pathol. 2022. PMID: 35698637 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349–361. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

