Phase 3 Study of Roxadustat to Treat Anemia in Non-Dialysis-Dependant CKD
- PMID: 34307976
- PMCID: PMC8258605
- DOI: 10.1016/j.ekir.2021.04.003
Phase 3 Study of Roxadustat to Treat Anemia in Non-Dialysis-Dependant CKD
Abstract
Introduction: Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that has demonstrated safety and efficacy versus placebo in phase III trials in patients with anemia of chronic kidney disease (CKD) who were not on dialysis (NDD).
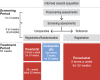
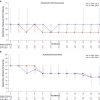
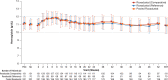
Methods: This was a phase III, active-controlled, multicenter, partially randomized, open-label study in Japanese patients with NDD CKD. Patients who had used recombinant human erythropoietin or darbepoetin alfa (DA) before conversion were randomized to roxadustat or DA (comparative arms). Patients who had used epoetin beta pegol before conversion were allocated to roxadustat (reference arm). The primary endpoint was change in average hemoglobin (Hb) level from baseline during the evaluation period (Weeks 18-24). Longer term efficacy and safety were evaluated in roxadustat-treated patients over 52 weeks.
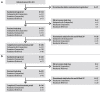
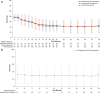
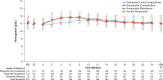
Results: In this study, 334 patients were randomized/allocated to receive treatment (n = 132, roxadustat [comparative]; n = 131, DA [comparative]; n = 71, roxadustat [reference]). The estimated difference between the roxadustat (comparative) and DA (comparative) groups in the least squares mean of change of average Hb levels of Weeks 18 to 24 from baseline was -0.07 g/dl, with the lower limit of 95% confidence interval of -0.23 g/dl, thereby confirming the noninferiority of roxadustat to DA. Common treatment-emergent adverse events (≥3% of patients in any treatment group) observed during the 24-week treatment period included nasopharyngitis, CKD, hyperkalemia, and hypertension.
Conclusion: Roxadustat maintained Hb within 10 to 12 g/dl in NDD CKD patients and was noninferior to DA. The safety profiles observed in this study are consistent with previous studies performed in this patient population.
Keywords: active comparator; anemia; chronic kidney disease; conversion; non–dialysis dependent; roxadustat.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
-
- Shih H.M., Wu C.J., Lin S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc. 2018;117:955–963. - PubMed
-
- Fishbane S., Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. 2018;71:423–435. - PubMed
-
- Iimori S., Naito S., Noda Y. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology (Carlton, Vic) 2015;20:601–608. - PubMed
LinkOut - more resources
Full Text Sources

