A Randomized Trial of Roxadustat in Anemia of Kidney Failure: SIERRAS Study
- PMID: 34307977
- PMCID: PMC8258588
- DOI: 10.1016/j.ekir.2021.04.007
A Randomized Trial of Roxadustat in Anemia of Kidney Failure: SIERRAS Study
Abstract
Introduction: Erythropoiesis-stimulating agents, standard of care for anemia of end-stage kidney disease, are associated with cardiovascular events. We evaluated the efficacy and safety of roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that stimulates erythropoiesis.
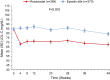
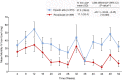
Methods: SIERRAS was a phase 3, randomized, open-label, active-controlled study enrolled adults on dialysis for end-stage kidney disease receiving erythropoiesis-stimulating agents for anemia. Patients were randomized (1:1) to thrice-weekly roxadustat or epoetin alfa. Doses were based on previous epoetin alfa dose and adjusted in the roxadustat arm to maintain hemoglobin at ∼11 g/dl during treatment. Epoetin alfa dosing was adjusted per US package insert. Primary efficacy endpoint was mean hemoglobin (g/dl) change from baseline averaged over weeks 28 to 52. Treatment-emergent adverse events were monitored.
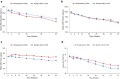
Results: Enrolled patients (roxadustat, n = 370 and epoetin alfa, n = 371) had similar mean (SD) baseline hemoglobin levels (10.30 [0.66] g/dl). Mean (SD) hemoglobin changes for weeks 28 to 52 were 0.39 (0.93) and -0.09 (0.84) in roxadustat and epoetin alfa, respectively. Roxadustat was noninferior (least squares mean difference: 0.48 [95% confidence interval: 0.37, 0.59]; P < 0.001) to epoetin alfa. Tolerability was comparable between treatments.
Conclusion: In end-stage kidney disease, roxadustat was noninferior to epoetin alfa in up to 52 weeks of treatment in this erythropoietin-stimulating agent conversion study. Roxadustat had an acceptable tolerability profile.
Keywords: dialysis; epoetin alfa; hemoglobin; kidney failure; roxadustat.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures




Comment in
-
Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors for the Treatment of Anemia in CKD: Additional Pieces of the Jigsaw Puzzle.Kidney Int Rep. 2021 Jun 23;6(7):1751-1754. doi: 10.1016/j.ekir.2021.05.017. eCollection 2021 Jul. Kidney Int Rep. 2021. PMID: 34308931 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System—United States. Version 7.1; 2020. https://nccd.cdc.gov/CKD/ Available at.
-
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. - PubMed
-
- Kidney Disease Outcomes Quality Initiative Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(suppl 3):S11–S145. - PubMed
LinkOut - more resources
Full Text Sources

