A Phase 3 Study of Enarodustat in Anemic Patients with CKD not Requiring Dialysis: The SYMPHONY ND Study
- PMID: 34307978
- PMCID: PMC8258589
- DOI: 10.1016/j.ekir.2021.04.037
A Phase 3 Study of Enarodustat in Anemic Patients with CKD not Requiring Dialysis: The SYMPHONY ND Study
Abstract
Introduction: Enarodustat (JTZ-951) is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that might be a new therapeutic approach for managing anemia in patients with chronic kidney disease (CKD). We evaluated the efficacy (noninferiority to darbepoetin alfa [DA]) and safety of enarodustat in Japanese anemic patients with CKD not requiring dialysis.
Methods: Erythropoiesis-stimulating agent (ESA)-naïve patients and ESA-treated patients were randomized at a 1:1 ratio to receive enarodustat orally once daily or DA subcutaneously every 2 or 4 weeks for 24 weeks, respectively. Subjects in each arm had dose adjustments every 4 weeks to maintain their hemoglobin (Hb) level within the target range (10 to 12 g/dl). The primary endpoint was the difference in the mean Hb level between arms during the evaluation period defined as weeks 20 to 24 (noninferiority margin: -0.75 g/dl).
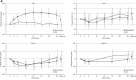
Results: The mean Hb level during the evaluation period in the enarodustat arm was 10.96 g/dl (95% confidence interval [CI]: 10.84 to 11.07 g/dl) with a difference of 0.09 g/dl (95% CI: -0.07 to 0.26 g/dl) between arms, establishing its noninferiority to DA. Nearly 90% of subjects in both arms maintained a mean Hb level within the target range. Compared with DA, enarodustat was associated with decreased hepcidin and ferritin, and increased total iron-binding capacity. There were no apparent differences in the incidence of adverse events between arms (65.4% [enarodustat], 82.6% [DA]).
Conclusions: The efficacy of enarodustat was comparable to DA in anemic patients with CKD not requiring dialysis. No new safety concerns were identified compared with DA.
Keywords: anemia in chronic kidney disease; comparative study; enarodustat; hepcidin; hypoxia-inducible factor prolyl hydroxylase inhibitor.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures



Comment in
-
Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors for the Treatment of Anemia in CKD: Additional Pieces of the Jigsaw Puzzle.Kidney Int Rep. 2021 Jun 23;6(7):1751-1754. doi: 10.1016/j.ekir.2021.05.017. eCollection 2021 Jul. Kidney Int Rep. 2021. PMID: 34308931 Free PMC article. No abstract available.
References
-
- Astor B.C., Muntner P., Levin A. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. - PubMed
-
- Scrutinio D., Passantino A., Santoro D. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. - PubMed
-
- Silverberg D.S., Wexler D., Blum M. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. - PubMed
-
- Silverberg D., Wexler D., Blum M. The cardio-renal anemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii7–viii12. - PubMed
LinkOut - more resources
Full Text Sources

