Endothelial Endothelin Receptor A Expression Is Associated With Podocyte Injury and Oxidative Stress in Patients With Focal Segmental Glomerulosclerosis
- PMID: 34307988
- PMCID: PMC8258598
- DOI: 10.1016/j.ekir.2021.04.013
Endothelial Endothelin Receptor A Expression Is Associated With Podocyte Injury and Oxidative Stress in Patients With Focal Segmental Glomerulosclerosis
Abstract
Introduction: The podocyte is thought to be the mainly affected cell type in focal segmental glomerulosclerosis (FSGS). However, recent studies have also indicated a role for glomerular endothelial cells and podocyte-endothelial crosstalk in FSGS development. An experimental model for podocyte injury showed that increased endothelin-1 (ET-1) signaling between podocytes and endothelial cells induces endothelial oxidative stress and subsequent podocyte loss. In the current study, we investigated endothelial endothelin receptor A (ETAR) expression in patients with FSGS and its association with podocyte injury and glomerular oxidative stress.
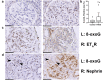
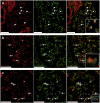
Methods: We selected 39 biopsy samples of patients with FSGS and 8 healthy control subjects, and stained them for ETAR, nephrin and 8-oxo-guanine, a DNA lesion caused by oxidative damage. Glomeruli with ETAR-positive endothelium and with nephrin loss were scored, and the 8-oxo-guanine-positive glomerular area was measured.
Results: The mean percentage of glomeruli with ETAR-positive endothelial cells in patients with FSGS was higher compared to that in healthy control subjects (52% vs. 7%; P < 0.001). The presence of glomerular ETAR-positive endothelium was strongly associated with nephrin loss both on the biopsy level (rho = 0.47; P < 0.01), as on the level of individual glomeruli (odds ratio = 2.0; P < 0.001). Moreover, glomeruli with ETAR-positive endothelium showed more 8-oxo-guanine-positive staining (1.9% vs. 2.4%; P = 0.037). Finally, 8-oxo-guanine positivity in glomeruli was associated with increased levels of proteinuria.
Conclusion: Taking together our findings, we show that ETAR is increased in glomerular endothelial cells of patients with FSGS and associated with podocyte damage and glomerular oxidative stress. These findings support the hypothesis that ET-1 signaling in glomerular endothelial cells contributes to disease development in patients with FSGS.
Keywords: crosstalk; endothelin receptor A; focal segmental glomerulosclerosis; glomerular endothelial cells; oxidative stress; podocytes.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures


Similar articles
-
Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis.J Clin Invest. 2014 Apr;124(4):1608-21. doi: 10.1172/JCI71195. Epub 2014 Mar 3. J Clin Invest. 2014. PMID: 24590287 Free PMC article.
-
Podocyte and endothelial injury in focal segmental glomerulosclerosis: an ultrastructural analysis.Virchows Arch. 2015 Oct;467(4):449-58. doi: 10.1007/s00428-015-1821-9. Epub 2015 Aug 13. Virchows Arch. 2015. PMID: 26266776 Free PMC article.
-
Glomerular endothelial cell injury and focal segmental glomerulosclerosis lesion in idiopathic membranous nephropathy.PLoS One. 2015 Apr 15;10(4):e0116700. doi: 10.1371/journal.pone.0116700. eCollection 2015. PLoS One. 2015. PMID: 25875837 Free PMC article.
-
The role of podocyte injury in the pathogenesis of focal segmental glomerulosclerosis.Ren Fail. 2000 Nov;22(6):663-84. doi: 10.1081/jdi-100101955. Ren Fail. 2000. PMID: 11104157 Review.
-
Focal segmental glomerulosclerosis; why does it occur segmentally?Pflugers Arch. 2017 Aug;469(7-8):983-988. doi: 10.1007/s00424-017-2023-x. Epub 2017 Jun 29. Pflugers Arch. 2017. PMID: 28664408 Review.
Cited by
-
Diverse Alterations of Glomerular Capillary Networks in Focal Segmental Glomerular Sclerosis.Kidney Int Rep. 2022 Mar 14;7(6):1229-1240. doi: 10.1016/j.ekir.2022.03.007. eCollection 2022 Jun. Kidney Int Rep. 2022. PMID: 35685313 Free PMC article.
-
Clinicopathological differences in focal segmental glomerulosclerosis depending on the accompanying pathophysiological conditions in renal allografts.Virchows Arch. 2023 Dec;483(6):809-819. doi: 10.1007/s00428-023-03703-6. Epub 2023 Nov 18. Virchows Arch. 2023. PMID: 37980299 Free PMC article.
-
Glomerular mTORC1 activation was associated with podocytes to endothelial cells communication in lupus nephritis.Lupus Sci Med. 2023 May;10(1):e000896. doi: 10.1136/lupus-2023-000896. Lupus Sci Med. 2023. PMID: 37147021 Free PMC article.
-
Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols.Front Immunol. 2023 Jul 21;14:1185317. doi: 10.3389/fimmu.2023.1185317. eCollection 2023. Front Immunol. 2023. PMID: 37545494 Free PMC article. Review.
-
Minimal Change Disease Is Associated With Endothelial Glycocalyx Degradation and Endothelial Activation.Kidney Int Rep. 2021 Dec 16;7(4):797-809. doi: 10.1016/j.ekir.2021.11.037. eCollection 2022 Apr. Kidney Int Rep. 2021. PMID: 35497798 Free PMC article.
References
-
- Jennette J.C., D'Agati V.D., Olson J.L. Wolters Kluwer Health; Philadelphia: 2014. Heptinstall’s Pathology of the Kidney.
-
- Buob D., Decambron M., Gnemmi V. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int. 2016;90:1321–1331. - PubMed
-
- Salvatore S.P., Reddi A.S., Chandran C.B. Collapsing glomerulopathy superimposed on diabetic nephropathy: insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol Dial Transplant. 2014;29:392–399. - PubMed
LinkOut - more resources
Full Text Sources

