MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia
- PMID: 34308304
- PMCID: PMC8257962
- DOI: 10.1016/j.eclinm.2021.100885
MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia
Abstract
Background: Binary prediction-models for outcome [death, cognition, presence and severity of cerebral palsy (CP)], using MRI and early clinical data applicable for individual outcome prediction have not been developed.
Methods: From Dec 1st 2006 until Dec 31st 2013, we recruited 178 infants into a population-based cohort with moderate or severe hypoxic-ischaemic encephalopathy (HIE) including postnatal collapse (PNC, n = 12) and additional diagnoses (n = 12) using CoolCap/TOBY-trial entry-criteria including depressed amplitude-integrated EEG (aEEG). Early clinical/biochemical variables and MRI scans (median day 8) were obtained in 168 infants. Injury severity was scored for cortex, basal ganglia/thalami (BGT), white matter (WM) and posterior limb of the internal capsule, summating to a total injury score (TIS, range 0-11). Outcome was categorized as adverse or favourable at 18-24 months from Bayley-III domains (cut-off 85) and neurological examination including CP classification.
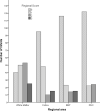
Findings: HIE and entry-aEEG severity were stable throughout the study. Outcome was favourable in 133/178 infants and adverse in 45/178: 17 died, 28 had low Cognition/Language scores, (including 9 with severe CP and 6 mild); seven had mild CP with favourable cognitive outcome. WMxBGT product scores and TIS were strong outcome predictors, and prediction improved when clinical/biochemical variables were added in binary logistic regression. The Positive Predictive Value for adverse outcome was 88%, increasing to 95% after excluding infants with PNC and additional diagnoses. Using WMxBGT in the regression predicted 8 of the 9 children with severe CP.
Interpretation: Binary logistic regression with WMxBGT or TIS and clinical variables gave excellent outcome prediction being 12% better than single variable cross-tabulation. Our MRI scoring and regression models are readily accessible and deserve investigation in other cohorts for group and individual prediction.
Funding: We thank the National Health Service (NHS) and our Universities and funders in UK and Norway: SPARKS, The Moulton Foundation, The Norwegian Research Council, The Lærdal Foundation for Acute Medicine and charitable donations for their support for cooling therapy.
Keywords: BGT, Basal ganglia/thalami; BIC, Bayesian information criterion; Basal ganglia and thalamus; Bayley-III; Bayley-III, Bayley Scales of Infant & Toddler Development 3rd edition; CLC, Cognitive and Language Composite from the Bayley-III scales; CP, Cerebral palsy; CX, Cortex; Cerebral palsy; Cortex; DWI, Diffusion-weighted imaging; GA, Gestational age; GMFCS, Gross Motor Function Classification System; HIE, Hypoxic-ischaemic encephalopathy; Hypoxic-ischaemic encephalopathy; ILEA, International League Against Epilepsy; IQR, Interquartile range; LDH72h, Lactate dehydrogenase close to 72h post-asphyxial event; LDHpeak, Highest LDH in the first 3 days; Logistic regression; MRI; MRI, Magnetic Resonance Imaging; Moderate or severe perinatal asphyxia; NPV, Negative Predictive Value; Neonatal seizures; Neurodevelopmental outcome; Outcome prediction; PA, Predictive Accuracy; PLIC, Posterior limb of the internal capsule; PNC, Postnatal collapse; PPV, Positive Predictive Value; Posterior limb of the internal capsule; RCT, Randomised controlled trial; Se, Sensitivity; Sp, Specificity; T1 and T2; TH, Therapeutic hypothermia; TIS, Total injury score; Therapeutic hypothermia; WMxBGT, Product of white matter and basal ganglia/thalami scores; White matter; aEEG, amplitude integrated electroencephalography; h, hours; lactatehrs<5mmol, plasma lactate recovery time; m, months.
© 2021 The Authors.
Conflict of interest statement
M Karlsson declares patents Method of Determining Hypoxia and Testing System for Determining Hypoxia Induced Cellular Damage. All other authors have nothing to disclose.
Figures



References
-
- Gluckman P.D., Wyatt J.S., Azzopardi D., Ballard R., Edwards A.D., Ferriero D.M. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. /02/22 ed. 2005 Feb 19. - PubMed
-
- Azzopardi D.V., Strohm B., Edwards A.D., Dyet L., Halliday H.L., Juszczak E. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. /10/03 ed. 2009 Oct 1. - PubMed
-
- Rutherford M., Ramenghi L.A., Edwards A.D., Brocklehurst P., Halliday H., Levene M. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2009;9(1):39–45. /11/10 ed. 2010 Jan. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

