Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose
- PMID: 34308389
- PMCID: PMC8266543
- DOI: 10.1016/j.xcrm.2021.100359
Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose
Abstract
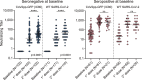
Massive vaccination offers great promise for halting the global COVID-19 pandemic. However, the limited supply and uneven vaccine distribution create an urgent need to optimize vaccination strategies. We evaluate SARS-CoV-2-specific antibody responses after Sputnik V vaccination of healthcare workers in Argentina, measuring IgG anti-spike titers and neutralizing capacity after one and two doses in a cohort of naive or previously infected volunteers. By 21 days after receiving the first dose of the vaccine, 94% of naive participants develop spike-specific IgG antibodies. A single Sputnik V dose elicits higher antibody levels and virus-neutralizing capacity in previously infected individuals than in naive ones receiving the full two-dose schedule. The high seroconversion rate after a single dose in naive participants suggests a benefit of delaying administration of the second dose to increase the number of people vaccinated. The data presented provide information for guiding public health decisions in light of the current global health emergency.
Keywords: COVID-19; COVIDAR IgG; SARS-CoV-2 vaccination; Sputnik V; WHO SARS-CoV-2 antibody International Standard; neutralizing antibodies; seroconversion.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. - PMC - PubMed
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Gam-COVID-Vac Vaccine Trial Group Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. - PMC - PubMed
-
- Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. N. Engl. J. Med. 2021;384:1372–1374. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

