Maintaining proteostasis under mechanical stress
- PMID: 34309183
- PMCID: PMC8339670
- DOI: 10.15252/embr.202152507
Maintaining proteostasis under mechanical stress
Abstract
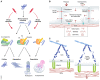
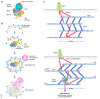
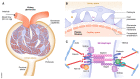
Cell survival, tissue integrity and organismal health depend on the ability to maintain functional protein networks even under conditions that threaten protein integrity. Protection against such stress conditions involves the adaptation of folding and degradation machineries, which help to preserve the protein network by facilitating the refolding or disposal of damaged proteins. In multicellular organisms, cells are permanently exposed to stress resulting from mechanical forces. Yet, for long time mechanical stress was not recognized as a primary stressor that perturbs protein structure and threatens proteome integrity. The identification and characterization of protein folding and degradation systems, which handle force-unfolded proteins, marks a turning point in this regard. It has become apparent that mechanical stress protection operates during cell differentiation, adhesion and migration and is essential for maintaining tissues such as skeletal muscle, heart and kidney as well as the immune system. Here, we provide an overview of recent advances in our understanding of mechanical stress protection.
Keywords: autophagy; chaperones; mechanobiology; proteostasis; signal transduction.
© 2021 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Adriaenssens E, Geuens T, Baets J, Echaniz‐Laguna A, Timmerman V (2017) Novel insights in the disease biology of mutant small heat shock proteins in neuromuscular diseases. Brain 140: 2541–2549 - PubMed
-
- Alon R, Dustin ML (2007) Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen‐presenting cells. Immunity 26: 17–27 - PubMed
-
- Altmann SM, Grünberg RG, Lenne P‐F, Ylänne J, Raae A, Herbert K, Saraste M, Nilges M, Hörber JKH (2002) Pathways and intermediates in forced unfolding of spectrin repeats. Structure 10: 1085–1096 - PubMed
-
- Arimura T, Ishikawa T, Nunoda S, Kawai S, Kimura A (2011) Dilated cardiomyopathy‐associated BAG3 mutations impair Z‐disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat 32: 1481–1491 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

