TROIKA-1: A double-blind, randomized, parallel group, study aimed to demonstrate the equivalent pharmacokinetic profile of HD201, a potential biosimilar candidate to trastuzumab, versus EU-Herceptin® and US-Herceptin® in healthy male subjects
- PMID: 34309241
- PMCID: PMC8311913
- DOI: 10.1002/prp2.839
TROIKA-1: A double-blind, randomized, parallel group, study aimed to demonstrate the equivalent pharmacokinetic profile of HD201, a potential biosimilar candidate to trastuzumab, versus EU-Herceptin® and US-Herceptin® in healthy male subjects
Abstract
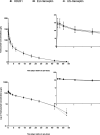
Prestige Biopharma Ltd (Singapore) has developed HD201, a proposed biosimilar to reference product trastuzumab. As a part of the stepwise approach to ensure comparability between the biosimilar candidate and the reference medicinal product, a phase I study in healthy subjects was conducted to demonstrate the pharmacokinetic (PK) equivalence (NCT03776240). The primary objective of the study was to demonstrate (PK) equivalence of HD201, EU-Herceptin® , and US-Herceptin® given at 6 mg/kg as a 90-min i.v. infusion to healthy male subjects. A pairwise comparisons based on the primary endpoint AUC0-inf and secondary PK endpoints, AUC0-last and Cmax were undertaken. PK equivalence was to be concluded if the 90% confidence interval (CI) for the ratio of geometric means for each criterion were within the equivalence margin of 80% to 125%. Secondary objectives included assessment of other PK parameters, safety, tolerability, and immunogenicity in the three arms. A total of 105 healthy male subjects (35/treatment) were randomized in this study. The 90% CI for the ratios of AUC0-inf , Cmax and AUC0-last , were within 80%-125% for the comparisons of HD201 to EU-Herceptin® or US-Herceptin® and EU-Herceptin® to US-Herceptin® . The frequency of subjects with TEAEs of special interest was slightly lower in the HD201 group (20.0%) compared to the other treatment groups (EU-Herceptin® : 34.3%; US-Herceptin® : 31.4%). Only 1 subject (EU-Herceptin® group) developed anti-drug antibodies prior to dosing. Overall, HD201 demonstrates PK similarity to both EU-Herceptin® and US-Herceptin® . The three study drugs also demonstrated similar safety profiles.
Keywords: biosimilar; pharmacokinetic; trastuzumab.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
Martin Demarchi, Pierre Coliat, Xavier Pivot, Kristi Mclendon, and Alexandre Detappe do not have any conflicts of interest for this article.
Jocelyn Chung Shii Hii, Peggy Feyaerts, Felicia Ang, and Litha Jaison were employees of Prestige Biopharma Ltd Singapore Michael Jinwoo Kim, Lisa Soyeon Park, were employees of Prestige Biopharma Ltd Singapore and Prestige Biologics Co Ltd, Korea.
Marie Paule Derde and Filip Deforce were employees by DICE Ltd which had a memorandum of understanding with Prestige Biopharma Ltd.
Figures

References
-
- Hayes DF. HER2 and breast cancer ‐ a phenomenal success story. N Engl J Med. 2019;381(13):1284‐1286. - PubMed
-
- Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Eng J Med. 2005;353(16):1734‐1736. - PubMed
-
- Agencies UEM . Scientific considerations in demonstrating biosimilarity to a reference product, Guidance for Industry. http://wwwfdagov/downloads/drugs/guidanceComplianceRegulatoryInformation.... 2015.
-
- Agency EM . Guideline on similar biological products containing biotechnology‐derived proteins as active sustance: non‐clinical and clinical issues. http://wwwemaeuropaeu/docs/en_GB/document_library/Scientif_guideline/201.... 2015.
-
- Pivot X, Deslypere JP, Park LS, Kim MJ, Lee W, Lee J. A Randomized phase I study comparing the pharmacokinetics of hd201, a trastuzumab biosimilar, with European union‐sourced herceptin. Clin Ther. 2018;40(3):396‐405 e4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

