DNA Electrochemistry: Charge-Transport Pathways through DNA Films on Gold
- PMID: 34309382
- PMCID: PMC9285625
- DOI: 10.1021/jacs.1c04713
DNA Electrochemistry: Charge-Transport Pathways through DNA Films on Gold
Abstract
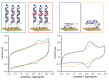
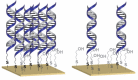
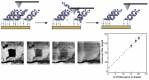
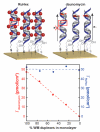
Over the past 25 years, collective evidence has demonstrated that the DNA base-pair stack serves as a medium for charge transport chemistry in solution and on DNA-modified gold surfaces. Since this charge transport depends sensitively upon the integrity of the DNA base pair stack, perturbations in base stacking, as may occur with DNA base mismatches, lesions, and protein binding, interrupt DNA charge transport (DNA CT). This sensitivity has led to the development of powerful DNA electrochemical sensors. Given the utility of DNA electrochemistry for sensing and in response to recent literature, we describe critical protocols and characterizations necessary for performing DNA-mediated electrochemistry. We demonstrate DNA electrochemistry with a fully AT DNA sequence using a thiolated preformed DNA duplex and distinguish this DNA-mediated chemistry from that of electrochemistry of largely single-stranded DNA adsorbed to the surface. We also demonstrate the dependence of DNA CT on a fully stacked duplex. An increase in the percentage of mismatches within the DNA monolayer leads to a linear decrease in current flow for a DNA-bound intercalator, where the reaction is DNA-mediated; in contrast, for ruthenium hexammine, which binds electrostatically to DNA and the redox chemistry is not DNA-mediated, there is no effect on current flow with mismatches. We find that, with DNA as a well hybridized duplex, upon assembly, a DNA-mediated pathway facilitates the electron transfer between a well coupled redox probe and the gold surface. Overall, this report highlights critical points to be emphasized when utilizing DNA electrochemistry and offers explanations and controls for analyzing confounding results.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

