Limited Expansion of Human Hepatocytes in FAH/ RAG2-Deficient Swine
- PMID: 34309416
- PMCID: PMC8892989
- DOI: 10.1089/ten.TEA.2021.0057
Limited Expansion of Human Hepatocytes in FAH/ RAG2-Deficient Swine
Abstract
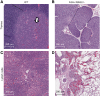
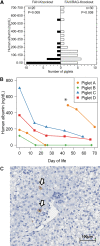
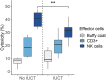
The mammalian liver's regenerative ability has led researchers to engineer animals as incubators for expansion of human hepatocytes. The expansion properties of human hepatocytes in immunodeficient mice are well known. However, little has been reported about larger animals that are more scalable and practical for clinical purposes. Therefore, we engineered immunodeficient swine to support expansion of human hepatocytes and identify barriers to their clinical application. Immunodeficient swine were engineered by knockout of the recombinase-activating gene 2 (RAG2) and fumarylacetoacetate hydrolase (FAH). Immature human hepatocytes (ihHCs) were injected into fetal swine by intrauterine cell transplantation (IUCT) at day 40 of gestation. Human albumin was measured as a marker of engraftment. Cytotoxicity against ihHCs was measured in transplanted piglets and control swine. We initially detected higher levels of human albumin in cord blood of newborn FAH/RAG2-deficient (FR) pigs compared with immunocompetent controls (196.26 ng/dL vs. 39.29 ng/dL, p = 0.008), indicating successful engraftment of ihHCs after IUCT and adaptive immunity in the fetus. Although rare hepatocytes staining positive for human albumin were observed, levels of human albumin did not rise after birth, but declined, suggesting rejection of xenografted ihHCs. Cytotoxicity against ihHCs increased after birth by 3.8% (95% CI: [2.1%-5.4%], p < 0.001) and inversely correlated with declining levels of human albumin (p = 2.1 × 10-5, R2 = 0.17). Circulating numbers of T cells and B cells were negligible in FR pigs. However, circulating natural killer (NK) cells exerted cytotoxicity against ihHCs. NK cell activity was lower in immunodeficient piglets after IUCT than in naive controls (30.4% vs. 40.1%, p = 0.011, 95% CI for difference [2.7%-16.7%]). In conclusion, ihHCs were successfully engrafted in FR swine after IUCT. NK cells were a significant barrier to expansion of hepatocytes. New approaches are needed to overcome this hurdle and allow large-scale expansion of human hepatocytes in immunodeficient swine. Impact statement There is currently a need for robust expansion of human hepatocytes. We describe an immunodeficient swine model into which we engrafted immature human hepatocytes (ihHCs). We identified the mechanism of the eventual graft rejection by the intact NK cell population, which has not been previously shown to have a significant role in xenograft rejection. By both improving engraftment and reducing NK cell-mediated cytotoxicity toward the graft through intrauterine cell transfer, we confirmed the presence of residual adaptive immunity in this model of immunodeficiency and the ability to induce hyposensitization in the NK cell population by taking advantage of the fetal microenvironment.
Keywords: animal model; genetic immunodeficiency; hepatocyte transplant; hereditary tyrosinemia; natural killer cell.
Conflict of interest statement
S.N., R.H., and J.L. are inventors of FAH-deficient swine. The FAH-deficient swine patent is owned by Mayo Clinic and Oregon Health and Science University and licensed to Cytotheryx (Rochester, MN). J.L. is a board member of Cytotheryx.
Figures


References
-
- Kobayashi, T., Yamaguchi, T., Hamanaka, S., et al. . Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787, 2010. - PubMed
-
- Ogle, B.M., Butters, K.A., Plummer, T.B., et al. . Spontaneous fusion of cells between species yields transdifferentiation and retroviral transfer in vivo. FASEB J 18, 548, 2004. - PubMed
-
- Ogle, B.M., Cascalho, M., and Platt, J.L.. Biological implications of cell fusion. Nature reviews. Mol Cell Biol 6, 567, 2005. - PubMed
-
- Grompe, M. Fah knockout animals as models for therapeutic liver repopulation. Adv Exp Med Biol 959, 215, 2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
