Comparison of HIV Screening Strategies in the Emergency Department: A Randomized Clinical Trial
- PMID: 34309668
- PMCID: PMC8314142
- DOI: 10.1001/jamanetworkopen.2021.17763
Comparison of HIV Screening Strategies in the Emergency Department: A Randomized Clinical Trial
Abstract
Importance: The National HIV Strategic Plan for the US recommends HIV screening in emergency departments (EDs). The most effective approach to ED-based HIV screening remains unknown.
Objective: To compare strategies for HIV screening when integrated into usual ED practice.
Design, setting, and participants: This randomized clinical trial included patients visiting EDs at 4 US urban hospitals between April 2014 and January 2016. Patients included were ages 16 years or older, not critically ill or mentally altered, not known to have an HIV positive status, and with an anticipated length of stay 30 minutes or longer. Data were analyzed through March 2021.
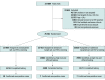
Interventions: Consecutive patients underwent concealed randomization to either nontargeted screening, enhanced targeted screening using a quantitative HIV risk prediction tool, or traditional targeted screening as adapted from the Centers for Disease Control and Prevention. Screening was integrated into clinical practice using opt-out consent and fourth-generation antigen-antibody assays.
Main outcomes and measures: New HIV diagnoses using intention-to-treat analysis, absolute differences, and risk ratios (RRs).
Results: A total of 76 561 patient visits were randomized; median (interquartile range) age was 40 (28-54) years, 34 807 patients (51.2%) were women, and 26 776 (39.4%) were Black, 22 131 (32.6%) non-Hispanic White, and 14 542 (21.4%) Hispanic. A total of 25 469 were randomized to nontargeted screening; 25 453, enhanced targeted screening; and 25 639, traditional targeted screening. Of the nontargeted group, 6744 participants (26.5%) completed testing and 10 (0.15%) were newly diagnosed; of the enhanced targeted group, 13 883 participants (54.5%) met risk criteria, 4488 (32.3%) completed testing, and 7 (0.16%) were newly diagnosed; and of the traditional targeted group, 7099 participants (27.7%) met risk criteria, 3173 (44.7%) completed testing, and 7 (0.22%) were newly diagnosed. When compared with nontargeted screening, targeted strategies were not associated with a higher rate of new diagnoses (enhanced targeted and traditional targeted combined: difference, -0.01%; 95% CI, -0.04% to 0.02%; RR, 0.7; 95% CI, 0.30 to 1.56; P = .38; and enhanced targeted only: difference, -0.01%; 95% CI, -0.04% to 0.02%; RR, 0.70; 95% CI, 0.27 to 1.84; P = .47).
Conclusions and relevance: Targeted HIV screening was not superior to nontargeted HIV screening in the ED. Nontargeted screening resulted in significantly more tests performed, although all strategies identified relatively low numbers of new HIV diagnoses.
Trial registration: ClinicalTrials.gov Identifier: NCT01781949.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention . Ending the HIV Epidemic: A Plan for America. Published July 30, 2020. Accessed April 1, 2021. https://www.cdc.gov/endhiv/docs/ending-HIV-epidemic-overview-508.pdf
-
- Centers for Disease Control and Prevention . HIV Basic Statistics. Published July 1, 2020. Accessed April 1, 2021. https://www.cdc.gov/hiv/basics/statistics.html
-
- Branson BM, Handsfield HH, Lampe MA, et al. ; Centers for Disease Control and Prevention . Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

