Correlation Between Surrogate End Points and Overall Survival in a Multi-institutional Clinicogenomic Cohort of Patients With Non-Small Cell Lung or Colorectal Cancer
- PMID: 34309669
- PMCID: PMC8314138
- DOI: 10.1001/jamanetworkopen.2021.17547
Correlation Between Surrogate End Points and Overall Survival in a Multi-institutional Clinicogenomic Cohort of Patients With Non-Small Cell Lung or Colorectal Cancer
Abstract
Importance: Contemporary observational cancer research requires associating genomic biomarkers with reproducible end points; overall survival (OS) is a key end point, but interpretation can be challenging when multiple lines of therapy and prolonged survival are common. Progression-free survival (PFS), time to treatment discontinuation (TTD), and time to next treatment (TTNT) are alternative end points, but their utility as surrogates for OS in real-world clinicogenomic data sets has not been well characterized.
Objective: To measure correlations between candidate surrogate end points and OS in a multi-institutional clinicogenomic data set.
Design, setting, and participants: A retrospective cohort study was conducted of patients with non-small cell lung cancer (NSCLC) or colorectal cancer (CRC) whose tumors were genotyped at 4 academic centers from January 1, 2014, to December 31, 2017, and who initiated systemic therapy for advanced disease. Patients were followed up through August 31, 2020 (NSCLC), and October 31, 2020 (CRC). Statistical analyses were conducted on January 5, 2021.
Exposures: Candidate surrogate end points included TTD; TTNT; PFS based on imaging reports only; PFS based on medical oncologist ascertainment only; PFS based on either imaging or medical oncologist ascertainment, whichever came first; and PFS defined by a requirement that both imaging and medical oncologist ascertainment have indicated progression.
Main outcomes and measures: The primary outcome was the correlation between candidate surrogate end points and OS.
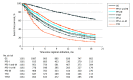
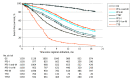
Results: There were 1161 patients with NSCLC (648 women [55.8%]; mean [SD] age, 63 [11] years) and 1150 with CRC (647 men [56.3%]; mean [SD] age, 54 [12] years) identified for analysis. Progression-free survival based on both imaging and medical oncologist documentation was most correlated with OS (NSCLC: ρ = 0.76; 95% CI, 0.73-0.79; CRC: ρ = 0.73; 95% CI, 0.69-0.75). Time to treatment discontinuation was least associated with OS (NSCLC: ρ = 0.45; 95% CI, 0.40-0.50; CRC: ρ = 0.13; 95% CI, 0.06-0.19). Time to next treatment was modestly associated with OS (NSCLC: ρ = 0.60; 0.55-0.64; CRC: ρ = 0.39; 95% CI, 0.32-0.46).
Conclusions and relevance: This cohort study suggests that PFS based on both a radiologist and a treating oncologist determining that a progression event has occurred was the surrogate end point most highly correlated with OS for analysis of observational clinicogenomic data.
Conflict of interest statement
Figures
Comment in
-
Assessing Clinical Outcomes in a Data-Rich World-A Reality Check on Real-World Data.JAMA Netw Open. 2021 Jul 1;4(7):e2117826. doi: 10.1001/jamanetworkopen.2021.17826. JAMA Netw Open. 2021. PMID: 34309673 No abstract available.
References
-
- AACR Project GENIE Consortium . AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818-831. doi:10.1158/2159-8290.CD-17-0151 - DOI - PMC - PubMed
-
- Chang E, Gong Y, Weinstock C, et al. . FDA pooled analysis of time to treatment discontinuation (TTD) in frontline advanced renal cell carcinoma trials. J Clin Oncol. 2020;38(15_suppl):5081. doi:10.1200/JCO.2020.38.15_suppl.5081 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

