Glucagon-Like Peptide 1 Receptor Agonist Usage in Type 2 Diabetes in Primary Care for the UK and Beyond: A Narrative Review
- PMID: 34309808
- PMCID: PMC8312211
- DOI: 10.1007/s13300-021-01116-9
Glucagon-Like Peptide 1 Receptor Agonist Usage in Type 2 Diabetes in Primary Care for the UK and Beyond: A Narrative Review
Abstract
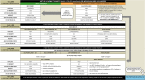
The scientific landscape of treatments for type 2 diabetes (T2D) has changed rapidly in the last decade with newer treatments becoming available. However, a large proportion of people with T2D are not able to achieve glycaemic goals because of clinical inertia. The majority of T2D management is in primary care, where clinicians (medical, nursing and pharmacist staff) play an important role in addressing patient needs and achieving treatment goals. However, management of T2D is challenging because of the heterogeneity of T2D and complexity of comorbidity, time constraints, guidance overload and the evolving treatments. Additionally, the current coronavirus disease pandemic poses additional challenges to the management of chronic diseases such as T2D, including routine access to patients for monitoring and communication. Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are a class of agents that have evolved rapidly in recent years. These agents act in a glucose-dependent manner to promote insulin secretion and inhibit glucagon secretion, as well as enhancing satiety and reducing hunger. As a result, they are effective treatment options for people with T2D, achieving glycated haemoglobin reductions, weight loss and potential cardiovascular benefit, as monotherapy or as add-on to other glucose-lowering therapies. However, given the complexity of managing T2D, it is important to equip primary care clinicians with clear information regarding efficacy, safety and appropriate positioning of GLP-1 RA therapies in clinical practice. This review provides a summary of clinical and real-world evidence along with practical guidance, with the aim of aiding primary care clinicians in the initiation and monitoring of GLP-1 RAs to help ensure that desired outcomes are realised. Furthermore, a benefit/risk tool has been developed on the basis of current available evidence and guidelines to support primary care clinicians in selecting individuals who are most likely to benefit from GLP-1 RA therapies, in addition to indicating clinical situations where caution is needed.
Keywords: Clinical guidance; Glucagon-like peptide 1 receptor agonist; Glucose-lowering medicines; Prescribing tools; Primary care; Risk/benefit; Therapy choice; Type 2 diabetes.
© 2021. The Author(s).
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

