Follicular helper and follicular regulatory T cell subset imbalance is associated with higher activated B cells and abnormal autoantibody production in primary anti-phospholipid syndrome patients
- PMID: 34309827
- PMCID: PMC8506124
- DOI: 10.1111/cei.13647
Follicular helper and follicular regulatory T cell subset imbalance is associated with higher activated B cells and abnormal autoantibody production in primary anti-phospholipid syndrome patients
Abstract
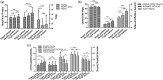
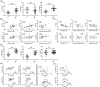
Primary anti-phospholipid antibody syndrome (pAPS) is a multi-organ autoimmune disease, and autoantibodies are involved in its pathogenesis. Follicular helper T cells (Tfh) and follicular regulatory T cells (Tfr) are critical for B cell maturation and antibody production, but their roles in pAPS remain unknown. We enrolled 32 pAPS patients and 23 healthy controls (HCs) and comprehensively analyzed circulating Tfh and Tfr, as well as their subsets, using flow cytometry. Clinical data including autoantibody levels were collected and their correlations with Tfh and Tfr subsets were analyzed. In addition, correlation analyses between B cell functional subsets and Tfh and Tfr were performed. Changes and potential effects of serum cytokines on Tfr and Tfh were further explored. We found the circulating Tfr was significantly decreased while Tfh and Tfh/Tfr ratios were increased in pAPS patients. Tfh2, inducible T cell co-stimulator (ICOS)+ programmed cell death 1 (PD-1)+ Tfh and Ki-67+ Tfh percentages were elevated, while CD45RA- forkhead box protein 3 (FoxP3)hi , Helios+ , T cell immunoglobulin and ITIM (TIGIT)+ and Ki-67+ Tfr percentages were decreased in pAPS patients. New memory B cells and plasmablasts were increased and altered B cell subsets and serum autoantibodies were positively correlated with Tfh, Tfh2, ICOS+ PD-1+ Tfh cells and negatively associated with Tfr, CD45RA- FoxP3hi Tfr and Helios+ Tfr cells. In addition, pAPS with LA/aCL/β2GPI autoantibodies showed lower functional Tfr subsets and higher activated Tfh subsets. Serum interleukin (IL)-4, IL-21, IL-12 and transforming growth factor (TGF)-β1 were up-regulated and associated with Tfh and Tfr subset changes. Our study demonstrates that imbalance of circulating Tfr and Tfh, as well as their functional subsets, is associated with abnormal autoantibody levels in pAPS, which may contribute to the pathogenesis of pAPS.
Keywords: B cell; anti-phospholipid antibody syndrome; autoantibody; follicular helper T cell; follicular regulatory T cell.
© 2021 British Society for Immunology.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures




References
-
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. - PubMed
-
- Arachchillage DRJ, Laffan M. Pathogenesis and management of antiphospholipid syndrome. Br J Haematol. 2017;178:181–95. - PubMed
-
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368:1033–44. - PubMed
-
- Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B‐lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82002214/National Natural Science Foundation of China
- 81871230/National Natural Science Foundation of China
- RDT 2020-01/Peking University People's Hospital Scientific Research Development Funds
- RDY 2019-15/Peking University People's Hospital Scientific Research Development Funds
- BMU2021PY007/Peking University Medicine Fund of Fostering Young Scholars' Scientific & Technological Innovation
LinkOut - more resources
Full Text Sources

