Molidustat for the treatment of anemia in Japanese patients undergoing peritoneal dialysis: a single-arm, open-label, phase 3 study
- PMID: 34310049
- PMCID: PMC9290504
- DOI: 10.1111/1744-9987.13713
Molidustat for the treatment of anemia in Japanese patients undergoing peritoneal dialysis: a single-arm, open-label, phase 3 study
Abstract
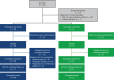
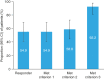
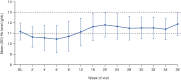
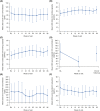
This 36-week, open-label, single-arm, phase 3 study investigated the safety and efficacy of molidustat in Japanese patients with renal anemia undergoing peritoneal dialysis. Molidustat was titrated every 4 weeks to maintain Hb levels within the target range (≥11.0 and <13.0 g/dL). The primary efficacy outcome was the responder rate, defined as the proportion of patients who met all of the following criteria: (1) mean Hb levels in the target range during the evaluation period (Weeks 30-36); (2) ≥50% of Hb values within the target range during the evaluation period; and (3) no rescue treatment before the end of the evaluation period. Overall, 51 patients received molidustat. The responder rate (95% CI) during the evaluation period was 54.9% (40.3, 68.9). Overall, 98.0% of patients experienced at least 1 adverse event during the study. No deaths were reported. Molidustat maintained Hb levels in the prespecified range in more than half of the patients and was well tolerated.
Keywords: anemia; erythropoiesis; renal dialysis; renal insufficiency.
© 2021 Bayer Yakuhin Ltd. Therapeutic Apheresis and Dialysis published by John Wiley & Sons Australia, Ltd on behalf of International Society for Apheresis, Japanese Society for Apheresis, and Japanese Society for Dialysis Therapy.
Conflict of interest statement
Tadao Akizawa received consulting and lecture fees from Bayer Yakuhin Ltd during the study. He also received consulting, lecture, or manuscript fees outside the submitted work from Astellas, GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical Co. Ltd, Kyowa Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd, Torii Pharmaceutical Co. Ltd, Sanwa Chemical Co. Ltd, Ono Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Tanabe Mitsubishi Co. Ltd, and Chugai Pharmaceutical Co. Ltd. Hiroyasu Yamamoto received consulting and lecture fees from Bayer Yakuhin Ltd during the study. Kiyoshi Nobori, Yoshimi Matsuda, Kentaro Taki, Yasuhiro Hayashi, and Takanori Hayasaki are employees of Bayer Yakuhin Ltd.
Figures

Similar articles
-
Molidustat for Renal Anemia in Nondialysis Patients Previously Treated with Erythropoiesis-Stimulating Agents: A Randomized, Open-Label, Phase 3 Study.Am J Nephrol. 2021;52(10-11):884-893. doi: 10.1159/000518072. Epub 2021 Sep 16. Am J Nephrol. 2021. PMID: 34569482 Clinical Trial.
-
Efficacy and Safety of Molidustat for Anemia in ESA-Naive Nondialysis Patients: A Randomized, Phase 3 Trial.Am J Nephrol. 2021;52(10-11):871-883. doi: 10.1159/000518071. Epub 2021 Sep 16. Am J Nephrol. 2021. PMID: 34569489 Clinical Trial.
-
Long-Term Efficacy and Safety of Molidustat for Anemia in Chronic Kidney Disease: DIALOGUE Extension Studies.Am J Nephrol. 2019;49(4):271-280. doi: 10.1159/000499111. Epub 2019 Mar 8. Am J Nephrol. 2019. PMID: 30852574 Free PMC article. Clinical Trial.
-
Molidustat for the treatment of renal anaemia in patients with dialysis-dependent chronic kidney disease: design and rationale of three phase III studies.BMJ Open. 2019 Jun 14;9(6):e026602. doi: 10.1136/bmjopen-2018-026602. BMJ Open. 2019. PMID: 31203241 Free PMC article. Clinical Trial.
-
Is there an established hemoglobin target range for patients undergoing chronic dialysis?Semin Dial. 2018 Jul;31(4):415-419. doi: 10.1111/sdi.12683. Epub 2018 Mar 6. Semin Dial. 2018. PMID: 29509320 Review.
Cited by
-
Evolving Strategies in the Treatment of Anaemia in Chronic Kidney Disease: The HIF-Prolyl Hydroxylase Inhibitors.Drugs. 2022 Nov;82(16):1565-1589. doi: 10.1007/s40265-022-01783-3. Epub 2022 Nov 9. Drugs. 2022. PMID: 36350500 Free PMC article. Review.
-
Lead Generation for a HIF Prolyl Hydroxylase Inhibitor: Discovery of (7-Hydroxy-[1,2,4]triazolo[1,5‑a]pyridine-8-carbonyl)glycine as a Lead Compound of Enarodustat.ACS Med Chem Lett. 2025 Apr 30;16(6):1124-1130. doi: 10.1021/acsmedchemlett.5c00172. eCollection 2025 Jun 12. ACS Med Chem Lett. 2025. PMID: 40529048
-
Simultaneous detection of three hypoxia-inducible factor stabilizers-molidustat, roxadustat, and vadadustat-in multiple keratinized matrices and its application in a doping context.Drug Test Anal. 2025 May;17(5):647-654. doi: 10.1002/dta.3771. Epub 2024 Jul 11. Drug Test Anal. 2025. PMID: 38992954 Free PMC article.
-
Current Status of Renal Anemia Pharmacotherapy-What Can We Offer Today.J Clin Med. 2021 Sep 15;10(18):4149. doi: 10.3390/jcm10184149. J Clin Med. 2021. PMID: 34575261 Free PMC article. Review.
-
Efficacy and safety of daprodustat in patients on peritoneal dialysis in the ASCEND-D trial.Nephrol Dial Transplant. 2025 Jun 30;40(7):1332-1341. doi: 10.1093/ndt/gfae273. Nephrol Dial Transplant. 2025. PMID: 39817409 Free PMC article. Clinical Trial.
References
-
- Kidney Disease . Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279–335.
-
- Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end‐stage renal disease. A collaborative meta‐analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–40. - PMC - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. - PubMed
-
- Nitta K, Goto S, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: survey methods, facility data, incidence, prevalence, and mortality. Renal Replace Ther. 2020;6(1):41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

