Direct-MS analysis of antibody-antigen complexes
- PMID: 34310051
- PMCID: PMC8595693
- DOI: 10.1002/pmic.202000300
Direct-MS analysis of antibody-antigen complexes
Abstract
In recent decades, antibodies (Abs) have attracted the attention of academia and the biopharmaceutical industry due to their therapeutic properties and versatility in binding a vast spectrum of antigens. Different engineering strategies have been developed for optimizing Ab specificity, efficacy, affinity, stability and production, enabling systematic screening and analysis procedures for selecting lead candidates. This quality assessment is critical but usually demands time-consuming and labor-intensive purification procedures. Here, we harnessed the direct-mass spectrometry (direct-MS) approach, in which the analysis is carried out directly from the crude growth media, for the rapid, structural characterization of designed Abs. We demonstrate that properties such as stability, specificity and interactions with antigens can be defined, without the need for prior purification.
Keywords: antibody antigen interactions; antibody deign; native mass spectrometry; protein stability.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest statement
The authors declare no competing interests.
Figures
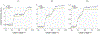


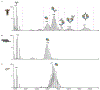
References
-
- Yang G; Velgos SN; Boddapati SP; Sierks MR, Probing Antibody-Antigen Interactions. Microbiol Spectr 2014, 2 (1), AID-0010-2013. - PubMed
-
- Zhou Q; Qiu H, The Mechanistic Impact of N-Glycosylation on Stability, Pharmacokinetics, and Immunogenicity of Therapeutic Proteins. J Pharm Sci 2019, 108 (4), 1366–1377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

