Myocardial Perfusion Defects in Hypertrophic Cardiomyopathy Mutation Carriers
- PMID: 34310159
- PMCID: PMC8475659
- DOI: 10.1161/JAHA.120.020227
Myocardial Perfusion Defects in Hypertrophic Cardiomyopathy Mutation Carriers
Abstract
Background Impaired myocardial blood flow (MBF) in the absence of epicardial coronary disease is a feature of hypertrophic cardiomyopathy (HCM). Although most evident in hypertrophied or scarred segments, reduced MBF can occur in apparently normal segments. We hypothesized that impaired MBF and myocardial perfusion reserve, quantified using perfusion mapping cardiac magnetic resonance, might occur in the absence of overt left ventricular hypertrophy (LVH) and late gadolinium enhancement, in mutation carriers without LVH criteria for HCM (genotype-positive, left ventricular hypertrophy-negative). Methods and Results A single center, case-control study investigated MBF and myocardial perfusion reserve (the ratio of MBF at stress:rest), along with other pre-phenotypic features of HCM. Individuals with genotype-positive, left ventricular hypertrophy-negative (n=50) with likely pathogenic/pathogenic variants and no evidence of LVH, and matched controls (n=28) underwent cardiac magnetic resonance. Cardiac magnetic resonance identified LVH-fulfilling criteria for HCM in 5 patients who were excluded. Individuals with genotype-positive, left ventricular hypertrophy-negative had longer indexed anterior mitral valve leaflet length (12.52±2.1 versus 11.55±1.6 mm/m2, P=0.03), lower left ventricular end-systolic volume (21.0±6.9 versus 26.7±6.2 mm/m2, P≤0.005) and higher left ventricular ejection fraction (71.9±5.5 versus 65.8±4.4%, P≤0.005). Maximum wall thickness was not significantly different (9.03±1.95 versus 8.37±1.2 mm, P=0.075), and no subject had significant late gadolinium enhancement (minor right ventricle‒insertion point late gadolinium enhancement only). Perfusion mapping demonstrated visual perfusion defects in 9 (20%) carriers versus 0 controls (P=0.011). These were almost all septal or near right ventricle insertion points. Globally, myocardial perfusion reserve was lower in carriers (2.77±0.83 versus 3.24±0.63, P=0.009), with a subendocardial:subepicardial myocardial perfusion reserve gradient (2.55±0.75 versus 3.2±0.65, P=<0.005; 3.01±0.96 versus 3.47±0.75, P=0.026) but equivalent MBF (2.75±0.82 versus 2.65±0.69 mL/g per min, P=0.826). Conclusions Regional and global impaired myocardial perfusion can occur in HCM mutation carriers, in the absence of significant hypertrophy or scarring.
Keywords: genetics; hypertrophic cardiomyopathy; quantitative perfusion mapping; sarcomere mutations carriers without hypertrophy.
Conflict of interest statement
None.
Figures

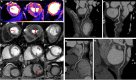
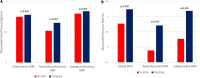
References
-
- Reant P, Captur G, Mirabel M, Nasis A, Sado DM, Maestrini V, Castelletti S, Manisty C, Herrey AS, Syrris P, et al. Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:1–8. DOI: 10.1186/s12968-015-0160-y. - DOI - PMC - PubMed
-
- Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–47. DOI: 10.1161/CIRCULATIONAHA.110.985812. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

