Plasma and Breast Milk Pharmacokinetics of Tenofovir Disoproxil Fumarate in Nursing Mother with Chronic Hepatitis B-Infant Pairs
- PMID: 34310204
- PMCID: PMC8448110
- DOI: 10.1128/AAC.01110-21
Plasma and Breast Milk Pharmacokinetics of Tenofovir Disoproxil Fumarate in Nursing Mother with Chronic Hepatitis B-Infant Pairs
Abstract
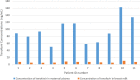
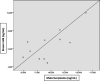
Tenofovir use is associated with lower risk of mother-to-infant transmission of the virus, and discontinuation of the treatment is not safe. However, the safety of the drug during pregnancy and breastfeeding is not clear. In this study, we aimed to determine the tenofovir concentration in plasma of mother-infant pairs along with breast milk in chronic hepatitis B patients during the lactation period. A total of 11 mother-infant pairs were enrolled in the study. All the mothers received tenofovir disoproxil fumarate (TDF) 245 mg/day for at least 1 month because of chronic hepatitis B infection. Maternal blood, breast milk, and infant blood samples were obtained concomitantly. Tenofovir concentrations were determined by liquid chromatography-tandem mass spectrometry. The median concentrations of tenofovir in maternal plasma and breast milk samples were 88.44 (interquartile range [IQR], 62.47 to 116.17) ng/ml and 6.69 (IQR, 4.88 to 7.03) ng/ml, respectively. Tenofovir concentrations were undetectable (<4 ng/ml) in all of the infant plasma samples. The ratio of tenofovir concentration in breast milk to that in maternal plasma was 0.07. Tenofovir disoproxil fumarate passes through the breast milk in a small amount. Infants had no detectable tenofovir level in their plasma. Our study suggests that tenofovir disoproxil fumarate treatment is safe during the breastfeeding period in chronic hepatitis B patients.
Keywords: breast milk; chronic hepatitis B; infant plasma; tenofovir.
Figures
References
-
- Lee H, Lok AS. 2020. Hepatitis B and pregnancy. InPost TW (ed), UpToDate. UpToDate, Waltham, MA.
-
- Liu J, Wang J, Jin D, Qi C, Yan TT, Cao F, Jin L, Tian Z, Guo D, Yuan N, Feng W, Zhang S, Zhao Y, Chen T. 2017. Hepatic flare after telbivudine withdrawal and efficacy of postpartum antiviral therapy for pregnancies with chronic hepatitis B virus. J Gastroenterol Hepatol 32:177–183. 10.1111/jgh.13436. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical